 | | DRUG: Hydroxychloroquine sulfate | |
| Entry |
|
|---|
| Name |
Hydroxychloroquine sulfate (JAN/USP);
Plaquenil (TN) |
|---|
| Product |
|
|---|
| Generic |
HYDROXYCHLOROQUINE SULFATE (A-S Medication Solutions), HYDROXYCHLOROQUINE SULFATE (A-S Medication Solutions), HYDROXYCHLOROQUINE SULFATE (A-S Medication Solutions), HYDROXYCHLOROQUINE SULFATE (A-S Medication Solutions), HYDROXYCHLOROQUINE SULFATE (Accord Healthcare), HYDROXYCHLOROQUINE SULFATE (American Health Packaging), HYDROXYCHLOROQUINE SULFATE (Amneal Pharmaceuticals NY LLC), HYDROXYCHLOROQUINE SULFATE (Amneal Pharmaceuticals NY LLC), HYDROXYCHLOROQUINE SULFATE (Aphena Pharma Solutions - Tennessee), HYDROXYCHLOROQUINE SULFATE (Aphena Pharma Solutions - Tennessee), HYDROXYCHLOROQUINE SULFATE (Aphena Pharma Solutions - Tennessee), HYDROXYCHLOROQUINE SULFATE (Asclemed USA), HYDROXYCHLOROQUINE SULFATE (AvPAK), HYDROXYCHLOROQUINE SULFATE (Bayshore Pharmaceuticals LLC), HYDROXYCHLOROQUINE SULFATE (Bryant Ranch Prepack), HYDROXYCHLOROQUINE SULFATE (Bryant Ranch Prepack), HYDROXYCHLOROQUINE SULFATE (Cardinal Health 107), HYDROXYCHLOROQUINE SULFATE (Cardinal Health 107), HYDROXYCHLOROQUINE SULFATE (Dr. Reddy's Laboratories), HYDROXYCHLOROQUINE SULFATE (Laurus Labs Limited), HYDROXYCHLOROQUINE SULFATE (Laurus Labs Limited), HYDROXYCHLOROQUINE SULFATE (Major Pharmaceuticals), HYDROXYCHLOROQUINE SULFATE (McKesson Corporation dba SKY Packaging), HYDROXYCHLOROQUINE SULFATE (NCS HealthCare of KY), HYDROXYCHLOROQUINE SULFATE (Northstar Rx LLC.), HYDROXYCHLOROQUINE SULFATE (NuCare Pharmaceuticals), HYDROXYCHLOROQUINE SULFATE (NuCare Pharmaceuticals), HYDROXYCHLOROQUINE SULFATE (Preferred Pharmaceuticals), HYDROXYCHLOROQUINE SULFATE (Quallent Pharmaceuticals Health LLC), HYDROXYCHLOROQUINE SULFATE (Rising Pharma Holdings), HYDROXYCHLOROQUINE SULFATE (Rising Pharma Holdings), HYDROXYCHLOROQUINE SULFATE (Sun Pharmaceutical Industries), HYDROXYCHLOROQUINE SULFATE (Teva Pharmaceuticals USA), HYDROXYCHLOROQUINE SULFATE (Zydus Lifesciences Limited), HYDROXYCHLOROQUINE SULFATE (Zydus Pharmaceuticals USA) |
|---|
| Formula |
C18H26ClN3O. H2SO4
|
|---|
| Exact mass |
433.1438
|
|---|
| Mol weight |
433.95
|
|---|
| Structure |
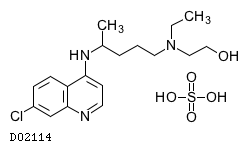
|
|---|
| Simcomp |
|
|---|
| Class |
Metabolizing enzyme substrate
DG02918 CYP2C8 substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
|
|---|
| Remark |
| Therapeutic category: | 3999 |
| Product (DG01015): | D02114<JP/US> |
|
|---|
| Efficacy |
Antimalarial |
|---|
| Disease |
Malaria [DS: H00361] Discoid lupus erythematosus [DS: H01595] Systemic lupus erythematosus [DS: H00080] Rheumatoid arthritis [DS: H00630] |
|---|
| Comment |
Aminoquinoline derivative
|
|---|
| Metabolism |
Enzyme: CYP2C8 [HSA: 1558], CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
P ANTIPARASITIC PRODUCTS, INSECTICIDES AND REPELLENTS
P01 ANTIPROTOZOALS
P01B ANTIMALARIALS
P01BA Aminoquinolines
P01BA02 Hydroxychloroquine
D02114 Hydroxychloroquine sulfate (JAN/USP) <JP/US>
USP drug classification [BR:br08302]
Antiparasitics
Antiprotozoals
Hydroxychloroquine
D02114 Hydroxychloroquine sulfate (JAN/USP)
Therapeutic category of drugs in Japan [BR:br08301]
3 Agents affecting metabolism
39 Other agents affecting metabolism
399 Miscellaneous
3999 Others
D02114 Hydroxychloroquine sulfate (JAN/USP)
Drug groups [BR:br08330]
Metabolizing enzyme substrate
DG02918 CYP2C8 substrate
DG01015 Hydroxychloroquine
D02114 Hydroxychloroquine sulfate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG01015 Hydroxychloroquine
D02114 Hydroxychloroquine sulfate
Antimicrobials [BR:br08307]
Antiparasitics
Antmalarials
Aminoquinoline
D02114 Hydroxychloroquine sulfate (JAN/USP) <JP/US>
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D02114
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D02114
Drug groups [BR:br08330]
Metabolizing enzyme substrate
DG02918 CYP2C8 substrate
DG01015 Hydroxychloroquine
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG01015 Hydroxychloroquine
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 28
1 S4a S 35.3817 -16.5587
2 O1d O 33.9825 -16.5587
3 O1d O 36.7810 -16.5587
4 O1d O 35.3817 -15.1594
5 O1d O 35.3817 -17.9579
6 N1b N 27.9259 -13.6586
7 C1c C 29.1334 -12.9646
8 C1b C 30.3405 -13.6644
9 C1a C 29.1392 -11.5648
10 C1b C 31.5540 -12.9703
11 C1b C 32.7552 -13.6703
12 N1c N 33.9685 -12.9821
13 C1b C 35.1757 -13.6819
14 C1b C 33.9743 -11.5822
15 C1b C 36.3833 -12.9878
16 C1a C 35.1816 -10.8882
17 C8y C 27.9236 -15.0841
18 C8y C 26.6906 -15.7937
19 C8y C 26.6886 -17.1897
20 N5x N 27.8966 -17.8895
21 C8x C 29.1297 -17.1800
22 C8x C 29.1317 -15.7839
23 C8x C 25.4815 -15.0956
24 C8x C 24.2725 -15.7937
25 C8y C 24.2725 -17.1897
26 C8x C 25.4815 -17.8878
27 X Cl 23.0615 -17.8890
28 O1a O 37.6097 -13.6988
BOND 28
1 1 2 1
2 1 3 1
3 1 4 2
4 1 5 2
5 6 7 1
6 7 8 1
7 7 9 1
8 8 10 1
9 10 11 1
10 11 12 1
11 12 13 1
12 12 14 1
13 13 15 1
14 14 16 1
15 6 17 1
16 17 18 1
17 18 19 2
18 19 20 1
19 20 21 2
20 21 22 1
21 17 22 2
22 18 23 1
23 23 24 2
24 24 25 1
25 25 26 2
26 26 19 1
27 25 27 1
28 15 28 1
|
|---|
|
» Japanese version » Back
|