 | | DRUG: Sulfadiazine, silver | |
| Entry |
|
|---|
| Name |
Sulfadiazine, silver (USP);
Sulfadiazine silver (JP18);
Silvadene (TN) |
|---|
| Product |
SILVADENE (A-S Medication Solutions), SILVADENE (Pfizer Laboratories Div Pfizer), SILVER SULFADIAZINE (A-S Medication Solutions), SILVER SULFADIAZINE (A-S Medication Solutions), SILVER SULFADIAZINE (A-S Medication Solutions), SILVER SULFADIAZINE (A-S Medication Solutions), SILVER SULFADIAZINE (Advanced Rx Pharmacy of Tennessee), SILVER SULFADIAZINE (Ascend Laboratories), SILVER SULFADIAZINE (Asclemed USA), SILVER SULFADIAZINE (Bryant Ranch Prepack), SILVER SULFADIAZINE (Bryant Ranch Prepack), SILVER SULFADIAZINE (Bryant Ranch Prepack), SILVER SULFADIAZINE (Bryant Ranch Prepack), SILVER SULFADIAZINE (Bryant Ranch Prepack), SILVER SULFADIAZINE (Bryant Ranch Prepack), SILVER SULFADIAZINE (Proficient Rx LP), SSD (A-S Medication Solutions), SSD CREAM (Dr Reddys Laboratories), SSD CREAM (NuCare Pharmaceuticals), SSD CREAM (Preferred Pharmaceuticals), SSD CREAM (RedPharm Drug), SSD CREAM (ST. MARY'S MEDICAL PARK PHARMACY) |
|---|
| Generic |
|
|---|
| Formula |
C10H9N4O2S. Ag
|
|---|
| Exact mass |
355.9497
|
|---|
| Mol weight |
357.14
|
|---|
| Structure |
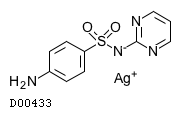
|
|---|
| Simcomp |
|
|---|
| Class |
Antibacterial
DG01787 Sulfonamide antibacterial
DG01785 Intermediate-acting sulfonamide
|
|---|
| Remark |
| Therapeutic category: | 2633 |
| Product (DG00599): | D00587<US> D00433<JP/US> |
|
|---|
| Efficacy |
Antibacterial (topical), Folic acid biosynthesis inhibitor |
|---|
| Comment |
Sulfonamide
|
|---|
| Target |
dihydropteroate synthase [KO: K00796] |
|---|
| Pathway |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07016 | Sulfonamide derivatives - sulfa drugs |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
D DERMATOLOGICALS
D06 ANTIBIOTICS AND CHEMOTHERAPEUTICS FOR DERMATOLOGICAL USE
D06B CHEMOTHERAPEUTICS FOR TOPICAL USE
D06BA Sulfonamides
D06BA01 Silver sulfadiazine
D00433 Sulfadiazine, silver (USP) <JP/US>
J ANTIINFECTIVES FOR SYSTEMIC USE
J01 ANTIBACTERIALS FOR SYSTEMIC USE
J01E SULFONAMIDES AND TRIMETHOPRIM
J01EC Intermediate-acting sulfonamides
J01EC02 Sulfadiazine
D00433 Sulfadiazine, silver (USP) <JP/US>
USP drug classification [BR:br08302]
Dermatological Agents
Dermatological Agents, Other
Wound Care Agents
Silver Sulfadiazine
D00433 Sulfadiazine, silver (USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
26 Epidermides
263 Suppurative dermatosis agents
2633 Sulfa drugs for external use
D00433 Sulfadiazine, silver (USP); Sulfadiazine silver (JP18)
Drug groups [BR:br08330]
Antibacterial
DG01787 Sulfonamide antibacterial
DG01785 Intermediate-acting sulfonamide
DG00599 Sulfadiazine
D00433 Sulfadiazine, silver
Antimicrobials [BR:br08307]
Antibacterials
Folic acid biosynthesis inhibitor
Intermediate-acting sulfonamide
D00433 Sulfadiazine, silver (USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00433 Sulfadiazine silver
Drug groups [BR:br08330]
Antibacterial
DG01787 Sulfonamide antibacterial
DG01785 Intermediate-acting sulfonamide
DG00599 Sulfadiazine
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 18
1 C8y C 24.2684 -17.0689
2 S4a S 25.4787 -16.3673
3 C8x C 24.2741 -18.4665
4 C8x C 23.0638 -16.3673
5 N1b N 26.6891 -17.0689 #-
6 O3c O 26.4610 -15.3792
7 O3c O 24.4788 -15.3792
8 C8x C 23.0638 -19.1682
9 C8x C 21.8534 -17.0572
10 C8y C 27.9052 -16.3673
11 C8y C 21.8477 -18.4665
12 N5x N 27.8995 -14.9639
13 N5x N 29.1097 -17.0689
14 N1a N 20.6373 -19.1622
15 C8x C 29.1097 -14.2623
16 C8x C 30.3200 -16.3732
17 C8x C 30.3259 -14.9639
18 Z Ag 26.6605 -18.9740 #+
BOND 18
1 1 2 1
2 1 3 2
3 1 4 1
4 2 5 1
5 2 6 2
6 2 7 2
7 3 8 1
8 4 9 2
9 5 10 1
10 8 11 2
11 10 12 2
12 10 13 1
13 11 14 1
14 12 15 1
15 13 16 2
16 15 17 2
17 9 11 1
18 16 17 1
|
|---|
|
» Japanese version » Back
|