| Entry |
|
|---|
| Name |
Alendronate sodium (USP);
Alendronate sodium hydrate (JP18);
Binosto (TN);
Fosamax (TN) |
|---|
| Product |
|
|---|
| Generic |
ALENDRONATE (Hangzhou Minsheng Binjiang Pharmaceutical), ALENDRONATE (Marlex Pharmaceuticals), ALENDRONATE SODIUM (A-S Medication Solutions), ALENDRONATE SODIUM (A-S Medication Solutions), ALENDRONATE SODIUM (A-S Medication Solutions), ALENDRONATE SODIUM (A-S Medication Solutions), ALENDRONATE SODIUM (A-S Medication Solutions), ALENDRONATE SODIUM (ANI Pharmaceuticals), ALENDRONATE SODIUM (Amneal Pharmaceuticals of New York LLC), ALENDRONATE SODIUM (Aurobindo Pharma Limited), ALENDRONATE SODIUM (Cipla USA), ALENDRONATE SODIUM (EXELAN PHARMACEUTICALS), ALENDRONATE SODIUM (Hikma Pharmaceuticals USA), ALENDRONATE SODIUM (NorthStar Rx LLC), ALENDRONATE SODIUM (NuCare Pharmaceuticals), ALENDRONATE SODIUM (NuCare Pharmaceuticals), ALENDRONATE SODIUM (Preferred Pharmaceuticals), ALENDRONATE SODIUM (Proficient Rx LP), ALENDRONATE SODIUM (REMEDYREPACK), ALENDRONATE SODIUM (REMEDYREPACK), ALENDRONATE SODIUM (RedPharm Drug), ALENDRONATE SODIUM (RedPharm Drug), ALENDRONATE SODIUM (Rising Pharma Holdings) |
|---|
| Formula |
C4H12NO7P2. 3H2O. Na
|
|---|
| Exact mass |
325.0304
|
|---|
| Mol weight |
325.12
|
|---|
| Structure |
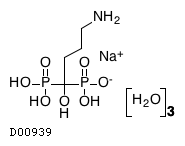
|
|---|
| Simcomp |
|
|---|
| Class |
Musculo-skeletal system agent
DG03232 Osteoporosis agent
DG01600 Bisphosphonate
|
|---|
| Remark |
| Therapeutic category: | 3999 |
| Product (DG00783): | D00939<JP/US> |
| Product (mixture): | D10841<US> |
|
|---|
| Efficacy |
Antiresorptive, Osteoporosis agent, Farnesylpyrophosphate synthetase inhibitor |
|---|
| Disease |
Osteoporosis [DS: H01593] Glucocorticoid-induced osteoporosis [DS: H01709] Paget's disease of bone [DS: H00437] |
|---|
| Comment |
Bisphosphonate
|
|---|
| Target |
|
|---|
| Pathway |
| hsa00900 | Terpenoid backbone biosynthesis |
|
|---|
| Interaction |
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
M MUSCULO-SKELETAL SYSTEM
M05 DRUGS FOR TREATMENT OF BONE DISEASES
M05B DRUGS AFFECTING BONE STRUCTURE AND MINERALIZATION
M05BA Bisphosphonates
M05BA04 Alendronic acid
D00939 Alendronate sodium (USP) <JP/US>
USP drug classification [BR:br08302]
Metabolic Bone Disease Agents
Bisphosphonates
Alendronate
D00939 Alendronate sodium (USP)
Therapeutic category of drugs in Japan [BR:br08301]
3 Agents affecting metabolism
39 Other agents affecting metabolism
399 Miscellaneous
3999 Others
D00939 Alendronate sodium (USP); Alendronate sodium hydrate (JP18)
Drug groups [BR:br08330]
Musculo-skeletal system agent
DG03232 Osteoporosis agent
DG01600 Bisphosphonate
DG00783 Alendronic acid
D00939 Alendronate sodium
Drug classes [BR:br08332]
Musculo-skeletal system agent
DG03232 Osteoporosis agent
D00939 Alendronate sodium
Target-based classification of drugs [BR:br08310]
Enzymes
Transferases (EC2)
Alkyl/Aryl transferases
FDPS
D00939 Alendronate sodium (USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00939 Alendronate sodium hydrate
D00939 Alendronate sodium tablets
D00939 Alendronate sodium injection
Drug groups [BR:br08330]
Musculo-skeletal system agent
DG03232 Osteoporosis agent
DG01600 Bisphosphonate
DG00783 Alendronic acid
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 18
1 O0 O 29.9425 -25.6767
2 Z Na 26.3872 -22.7066 #+
3 P1b P 22.2600 -24.5000
4 C1d C 23.5900 -24.5000
5 C1b C 23.5900 -22.6100
6 O1a O 23.5900 -25.8300
7 P1b P 24.9900 -24.5000
8 O1c O 26.3200 -24.5000 #-
9 O1c O 20.8600 -24.5000
10 O1c O 22.2600 -23.1000
11 O1c O 24.9900 -23.1000
12 C1b C 24.7800 -21.9800
13 C1b C 24.7800 -20.5800
14 N1a N 25.9700 -19.8800
15 O1c O 22.2600 -25.8300
16 O1c O 24.9900 -25.8300
17 O0 O 29.9425 -25.6767
18 O0 O 29.9425 -25.6767
BOND 13
1 3 4 1
2 4 5 1
3 4 6 1
4 4 7 1
5 7 8 1
6 3 9 1
7 3 10 2
8 7 11 2
9 5 12 1
10 12 13 1
11 13 14 1
12 3 15 1
13 7 16 1
BRACKET 1 28.2800 -26.7400 28.2800 -24.7800
1 30.3100 -24.7800 30.3100 -26.7400
1 3
ORIGINAL 1 1
REPEAT 1 17 18
|
|---|