| Entry |
|
|---|
| Name |
Magnesium sulfate (USP);
Magnesium sulfate hydrate (JP18);
Magnesium sulfate heptahydrate;
Conclyte-Mg (TN) |
|---|
| Product |
MAGNESIUM SULFATE (Fresenius Kabi USA), MAGNESIUM SULFATE (Fresenius Kabi USA), MAGNESIUM SULFATE (Fresenius Kabi USA), MAGNESIUM SULFATE (Fresenius Kabi USA), MAGNESIUM SULFATE (HF Acquisition Co LLC), MAGNESIUM SULFATE (HF Acquisition Co LLC), MAGNESIUM SULFATE (HF Acquisition Co LLC), MAGNESIUM SULFATE (Henry Schein), MAGNESIUM SULFATE (Henry Schein), MAGNESIUM SULFATE (Medical Purchasing Solutions), MAGNESIUM SULFATE IN DEXTROSE (Hospira), MAGNESIUM SULFATE IN WATER (Hospira) |
|---|
| Generic |
50% MAGNESIUM SULFATE (HF Acquisition Co. LLC), MAGNESIUM SULFATE (Amneal Pharmaceuticals LLC), MAGNESIUM SULFATE (Baxter Healthcare Corporation), MAGNESIUM SULFATE (Fresenius Kabi USA), MAGNESIUM SULFATE (Fresenius Kabi USA), MAGNESIUM SULFATE (Gland Pharma Limited), MAGNESIUM SULFATE (HF Acquisition Co LLC), MAGNESIUM SULFATE (Henry Schein), MAGNESIUM SULFATE (Hospira), MAGNESIUM SULFATE (Hospira), MAGNESIUM SULFATE (Mylan Institutional LLC), MAGNESIUM SULFATE (Sagent Pharmaceuticals), MAGNESIUM SULFATE (Sun Pharmaceutical Industries), MAGNESIUM SULFATE IN 5% DEXTROSE (Fresenius Kabi USA), MAGNESIUM SULFATE IN 5% DEXTROSE (Fresenius Kabi USA), MAGNESIUM SULFATE IN DEXTROSE (B. Braun Medical), MAGNESIUM SULFATE IN DEXTROSE (Baxter Healthcare Corporation), MAGNESIUM SULFATE IN DEXTROSE (Mylan Institutional LLC), MAGNESIUM SULFATE IN DEXTROSE (WG Critical Care), MAGNESIUM SULFATE IN WATER (Athenex Pharmaceutical Division), MAGNESIUM SULFATE IN WATER (Avenacy), MAGNESIUM SULFATE IN WATER (B. Braun Medical), MAGNESIUM SULFATE IN WATER (WG Critical Care) |
|---|
| Formula |
SO4. 7H2O. Mg
|
|---|
| Exact mass |
246.0107
|
|---|
| Mol weight |
246.48
|
|---|
| Structure |
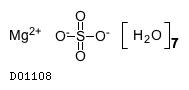
|
|---|
| Simcomp |
|
|---|
| Class |
Gastrointestinal agent
DG01770 Laxative
DG01979 Magnesium containing preparation
|
|---|
| Remark |
| Product (mixture): | D02019<JP> D04109<JP> D08711<JP> D11985<JP/US> |
|
|---|
| Efficacy |
Antiarrhythmic, Laxative, Replenisher (electrolyte) |
|---|
| Disease |
|
|---|
| Interaction |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
A ALIMENTARY TRACT AND METABOLISM
A06 DRUGS FOR CONSTIPATION
A06A DRUGS FOR CONSTIPATION
A06AD Osmotically acting laxatives
A06AD04 Magnesium sulfate
D01108 Magnesium sulfate (USP) <JP/US>
A12 MINERAL SUPPLEMENTS
A12C OTHER MINERAL SUPPLEMENTS
A12CC Magnesium
A12CC02 Magnesium sulfate
D01108 Magnesium sulfate (USP) <JP/US>
B BLOOD AND BLOOD FORMING ORGANS
B05 BLOOD SUBSTITUTES AND PERFUSION SOLUTIONS
B05X I.V. SOLUTION ADDITIVES
B05XA Electrolyte solutions
B05XA05 Magnesium sulfate
D01108 Magnesium sulfate (USP) <JP/US>
D DERMATOLOGICALS
D11 OTHER DERMATOLOGICAL PREPARATIONS
D11A OTHER DERMATOLOGICAL PREPARATIONS
D11AX Other dermatologicals
D11AX05 Magnesium sulfate
D01108 Magnesium sulfate (USP) <JP/US>
V VARIOUS
V04 DIAGNOSTIC AGENTS
V04C OTHER DIAGNOSTIC AGENTS
V04CC Tests for bile duct patency
V04CC02 Magnesium sulfate
D01108 Magnesium sulfate (USP) <JP/US>
USP drug classification [BR:br08302]
Electrolytes/Minerals/Metals/Vitamins
Electrolyte/ Mineral Replacement
Magnesium
D01108 Magnesium sulfate (USP)
Therapeutic category of drugs in Japan [BR:br08301]
1 Agents affecting nervous system and sensory organs
12 Agents affecting peripheral nervous system
124 Antispasmodics
1244 Magnesium salts
D01108 Magnesium sulfate (USP); Magnesium sulfate hydrate (JP18)
2 Agents affecting individual organs
23 Digestive organ agents
235 Purgatives, clysters
2355 Inorganic salts
D01108 Magnesium sulfate (USP); Magnesium sulfate hydrate (JP18)
Classification of Japanese OTC drugs [BR:br08313]
Agents for digestive organs
17 Cathartics
D01108 Magnesium sulfate (USP)
Risk category of Japanese OTC drugs [BR:br08312]
Third-class OTC drugs
Inorganic and organic chemicals
Magnesium sulfate hydrate
D01108 Magnesium sulfate (USP)
Drug groups [BR:br08330]
Gastrointestinal agent
DG01770 Laxative
D01108 Magnesium sulfate
DG01979 Magnesium containing preparation
D01108 Magnesium sulfate
Drug classes [BR:br08332]
Gastrointestinal agent
DG01770 Laxative
D01108 Magnesium sulfate
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D01108 Magnesium sulfate hydrate
D01108 Magnesium sulfate mixture
D01108 Magnesium sulfate injection
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 13
1 S4a S 22.0075 -19.5414
2 O1d O 22.0004 -18.1414
3 O1d O 22.0004 -20.9414
4 O1d O 23.4075 -19.5414 #-
5 O1d O 20.6075 -19.5414 #-
6 Z Mg 17.4222 -19.4642 #2+
7 O0 O 27.3000 -19.4600
8 O0 O 27.3000 -19.4600
9 O0 O 27.3000 -19.4600
10 O0 O 27.3000 -19.4600
11 O0 O 27.3000 -19.4600
12 O0 O 27.3000 -19.4600
13 O0 O 27.3000 -19.4600
BOND 4
1 1 3 2
2 1 4 1
3 1 5 1
4 1 2 2
BRACKET 1 25.2700 -20.3700 25.2700 -18.5500
1 27.8600 -18.5500 27.8600 -20.3700
1 7
ORIGINAL 1 7
REPEAT 1 8 9 10 11 12 13
|
|---|
|