| Entry |
|
|---|
| Name |
Leucovorin calcium (USP);
Calcium folinate (JP18);
Uzel (TN);
Wellcovorin (TN) |
|---|
| Product |
|
|---|
| Generic |
LEUCOVORIN CALCIUM (American Health Packaging), LEUCOVORIN CALCIUM (BluePoint Laboratories), LEUCOVORIN CALCIUM (BluePoint Laboratories), LEUCOVORIN CALCIUM (Epic Pharma LLC), LEUCOVORIN CALCIUM (Fresenius Kabi USA), LEUCOVORIN CALCIUM (Fresenius Kabi USA), LEUCOVORIN CALCIUM (Fresenius Kabi USA), LEUCOVORIN CALCIUM (Fresenius Kabi USA), LEUCOVORIN CALCIUM (Hikma Pharmaceuticals USA), LEUCOVORIN CALCIUM (Hikma Pharmaceuticals USA), LEUCOVORIN CALCIUM (Hikma Pharmaceuticals USA), LEUCOVORIN CALCIUM (Ingenus Pharmaceuticals), LEUCOVORIN CALCIUM (Leading Pharma), LEUCOVORIN CALCIUM (Major Pharmaceuticals), LEUCOVORIN CALCIUM (Meitheal Pharmaceuticals), LEUCOVORIN CALCIUM (Mylan Institutional LLC), LEUCOVORIN CALCIUM (Proficient Rx LP), LEUCOVORIN CALCIUM (Sagent Pharmaceuticals), LEUCOVORIN CALCIUM (Sagent Pharmaceuticals), LEUCOVORIN CALCIUM (Slate Run Pharmaceuticals), LEUCOVORIN CALCIUM (Teva Pharmaceuticals USA) |
|---|
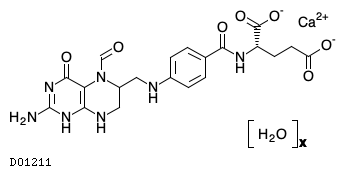
| Formula |
C20H21N7O7. Ca. xH2O
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Remark |
| Therapeutic category: | 3929 |
| Product (DG02073): | D01211<JP/US> |
|
|---|
| Efficacy |
Anti-anemic (folate deficiency), Antidote (antifolate), Antineoplastic (enhancer), Supplement (folic acid) |
|---|
| Disease |
|
|---|
| Comment |
Tetrahydrofolic acid [CPD: C00101] derivative
|
|---|
| Interaction |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
V VARIOUS
V03 ALL OTHER THERAPEUTIC PRODUCTS
V03A ALL OTHER THERAPEUTIC PRODUCTS
V03AF Detoxifying agents for antineoplastic treatment
V03AF03 Calcium folinate
D01211 Leucovorin calcium (USP) <JP/US>
USP drug classification [BR:br08302]
Antineoplastics
Antineoplastic Adjuncts/Mitigators
Leucovorin
D01211 Leucovorin calcium (USP)
Antineoplastics, Other
Antineoplastics, Miscellaneous
Leucovorin
D01211 Leucovorin calcium (USP)
Therapeutic category of drugs in Japan [BR:br08301]
3 Agents affecting metabolism
39 Other agents affecting metabolism
392 Antidotes
3929 Others
D01211 Leucovorin calcium (USP); Calcium folinate (JP18)
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D01211 Calcium folinate
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 36
1 C1c C 17.4741 -4.3941
2 C1b C 18.6628 -5.0934
3 C6a C 17.4741 -2.9956
4 C8y C 5.3771 -7.2610
5 N1y N 6.5659 -6.5618
6 C8y C 5.3771 -8.6595
7 C8y C 4.1185 -6.5618
8 C1y C 7.8245 -7.2610
9 N1x N 6.5659 -9.3588
10 N4x N 4.1185 -9.3588
11 N5x N 2.9298 -7.2610
12 O5x O 4.1185 -5.1633
13 C1x C 7.8245 -8.6595
14 C1b C 9.0132 -6.5618
15 C8y C 2.9298 -8.6595
16 N1a N 1.7410 -9.3588
17 N1b N 10.2019 -7.2610
18 C8y C 11.3907 -6.5618
19 C8x C 11.3907 -5.1633
20 C8x C 12.5794 -4.4640
21 C8y C 13.8380 -5.1633
22 C8x C 13.8380 -6.5618
23 C8x C 12.6493 -7.2610
24 C5a C 15.0268 -4.4640
25 N1b N 16.2854 -5.1633
26 O5a O 15.0268 -3.0656
27 O6a O 18.6628 -2.2964 #-
28 C1b C 19.8516 -4.3941
29 O6a O 16.2854 -2.2964
30 C6a C 21.0403 -5.0934
31 O6a O 22.2989 -4.3242 #-
32 O6a O 21.0403 -6.4919
33 C4a C 6.5659 -5.1633
34 O4a O 7.7546 -4.4640
35 Z Ca 20.6907 -2.7159 #2+
36 O0 O 19.1800 -10.5700
BOND 36
1 1 2 1
2 1 3 1 #Down
3 4 5 1
4 4 6 2
5 4 7 1
6 5 8 1
7 6 9 1
8 6 10 1
9 7 11 1
10 7 12 2
11 8 13 1
12 8 14 1
13 10 15 1
14 15 16 1
15 9 13 1
16 11 15 2
17 14 17 1
18 17 18 1
19 18 19 2
20 19 20 1
21 20 21 2
22 21 22 1
23 22 23 2
24 18 23 1
25 21 24 1
26 24 25 1
27 24 26 2
28 1 25 1
29 3 27 1
30 2 28 1
31 3 29 2
32 28 30 1
33 30 31 1
34 30 32 2
35 5 33 1
36 33 34 2
BRACKET 1 17.2200 -11.4800 17.2200 -9.5900
1 19.9500 -9.5900 19.9500 -11.4800
1 x
ORIGINAL 1 36
REPEAT 1
|
|---|
|