| Entry |
|
|---|
| Name |
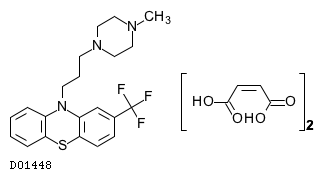
Trifluoperazine maleate (JAN);
Trifluoperazine (TN) |
|---|
| Formula |
C21H24F3N3S. (C4H4O4)2
|
|---|
| Exact mass |
639.1862
|
|---|
| Mol weight |
639.64
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
Neuropsychiatric agent
DG01478 Dopamine antagonist
DG01474 Dopamine D2-receptor antagonist
DG03200 Antipsychotic agent
DG01905 Phenothiazine antipsychotics
|
|---|
| Remark |
| Product (DG00877): | D00799<US> |
|
|---|
| Efficacy |
Antipsychotic, Dopamine D2 receptor antagonist |
|---|
| Comment |
Phenothiazine derivative
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07029 | Antipsychotics - phenothiazines |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
N NERVOUS SYSTEM
N05 PSYCHOLEPTICS
N05A ANTIPSYCHOTICS
N05AB Phenothiazines with piperazine structure
N05AB06 Trifluoperazine
D01448 Trifluoperazine maleate (JAN)
Drug groups [BR:br08330]
Neuropsychiatric agent
DG01478 Dopamine antagonist
DG01474 Dopamine D2-receptor antagonist
DG00877 Trifluoperazine
D01448 Trifluoperazine maleate
DG03200 Antipsychotic agent
DG01905 Phenothiazine antipsychotics
DG00877 Trifluoperazine
D01448 Trifluoperazine maleate
Target-based classification of drugs [BR:br08310]
G Protein-coupled receptors
Rhodopsin family
Dopamine
DRD2
D01448 Trifluoperazine maleate (JAN)
Drug groups [BR:br08330]
Neuropsychiatric agent
DG01478 Dopamine antagonist
DG01474 Dopamine D2-receptor antagonist
DG00877 Trifluoperazine
DG03200 Antipsychotic agent
DG01905 Phenothiazine antipsychotics
DG00877 Trifluoperazine
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 44
1 C8y C 17.5418 -18.2333
2 N1y N 18.7497 -17.5389
3 C8y C 17.5418 -19.6278
4 C8x C 16.3282 -17.5389
5 C8y C 19.9750 -18.2567
6 C1b C 18.7497 -16.1210
7 S2x S 18.7497 -20.3163
8 C8x C 16.3282 -20.3163
9 C8x C 15.1203 -18.2567
10 C8y C 19.9750 -19.6045
11 C8x C 21.1887 -17.5565
12 C1b C 19.9750 -15.4208
13 C8x C 15.1203 -19.6045
14 C8x C 21.1887 -20.3046
15 C8y C 22.4140 -18.2683
16 C1b C 19.9750 -14.0146
17 C8x C 22.4140 -19.5928
18 N1y N 21.1887 -13.3145
19 C1x C 21.2004 -11.9140
20 C1x C 22.3965 -14.0146
21 C1x C 22.4140 -11.2254
22 C1x C 23.6277 -13.3028
23 N1y N 23.6277 -11.9374
24 C1a C 24.8647 -11.2664
25 C1d C 23.6161 -17.5764
26 X F 24.7800 -16.8700
27 X F 24.2900 -18.7600
28 X F 22.8900 -16.3800
29 C2b C 32.6453 -16.0332
30 C2b C 31.0108 -16.0390
31 C6a C 33.3225 -17.2123
32 C6a C 30.3395 -17.2240
33 O6a O 32.6510 -18.3856
34 O6a O 34.6884 -17.1949
35 O6a O 28.9735 -17.2240
36 O6a O 31.0223 -18.3973
37 C2b C 32.6453 -16.0332
38 C2b C 31.0108 -16.0390
39 C6a C 30.3395 -17.2240
40 O6a O 28.9735 -17.2240
41 O6a O 31.0223 -18.3973
42 C6a C 33.3225 -17.2123
43 O6a O 32.6510 -18.3856
44 O6a O 34.6884 -17.1949
BOND 45
1 10 14 1
2 11 15 2
3 12 16 1
4 14 17 2
5 16 18 1
6 18 19 1
7 18 20 1
8 19 21 1
9 20 22 1
10 21 23 1
11 23 24 1
12 7 10 1
13 9 13 1
14 15 17 1
15 22 23 1
16 1 2 1
17 1 3 2
18 1 4 1
19 2 5 1
20 2 6 1
21 3 7 1
22 3 8 1
23 4 9 2
24 5 10 2
25 5 11 1
26 6 12 1
27 8 13 2
28 15 25 1
29 25 26 1
30 25 27 1
31 25 28 1
32 29 30 2
33 29 31 1
34 30 32 1
35 31 33 1
36 31 34 2
37 32 35 1
38 32 36 2
39 37 38 2
40 37 42 1
41 38 39 1
42 42 43 1
43 42 44 2
44 39 40 1
45 39 41 2
BRACKET 1 27.4400 -19.1800 27.4400 -15.1900
1 35.3500 -15.1900 35.3500 -19.1800
1 2
ORIGINAL 1 29 30 32 35 36 31 33 34
REPEAT 1 37 38 39 40 41 42 43 44
|
|---|