 | | DRUG: Sodium chloride | |
| Entry |
|
|---|
| Name |
Sodium chloride (JP18/USP);
Adsorbanac (TN) |
|---|
| Product |
0.9% SODIUM CHLORIDE (HF Acquisition Co LLC), 0.9% SODIUM CHLORIDE (HF Acquisition Co LLC), 0.9% SODIUM CHLORIDE (HF Acquisition Co LLC), 0.9% SODIUM CHLORIDE (HF Acquisition Co LLC), BACTERIOSTATIC SODIUM CHLORIDE (HF Acquisition Co LLC), BACTERIOSTATIC SODIUM CHLORIDE (HF Acquisition Co LLC), BACTERIOSTATIC SODIUM CHLORIDE (Henry Schein), BACTERIOSTATIC SODIUM CHLORIDE (Hospira), BACTERIOSTATIC SODIUM CHLORIDE (Medical Purchasing Solutions), BACTERIOSTATIC SODIUM CHLORIDE (ProPharma Distribution), SODIUM CHLORIDE (Asclemed USA), SODIUM CHLORIDE (B. Braun Medical), SODIUM CHLORIDE (B. Braun Medical), SODIUM CHLORIDE (B. Braun Medical), SODIUM CHLORIDE (B. Braun Medical), SODIUM CHLORIDE (B. Braun Medical), SODIUM CHLORIDE (B. Braun Medical), SODIUM CHLORIDE (B. Braun Medical), SODIUM CHLORIDE (Baxter Healthcare Company), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Baxter Healthcare Corporation), SODIUM CHLORIDE (Cardinal Health 107), SODIUM CHLORIDE (HF Acquisition Co LLC), SODIUM CHLORIDE (HF Acquisition Co LLC), SODIUM CHLORIDE (HF Acquisition Co LLC), SODIUM CHLORIDE (Henry Schein), SODIUM CHLORIDE (Hospira), SODIUM CHLORIDE (Hospira), SODIUM CHLORIDE (Hospira), SODIUM CHLORIDE (Hospira), SODIUM CHLORIDE (ICU Medical), SODIUM CHLORIDE (ICU Medical), SODIUM CHLORIDE (Liebel-Flarsheim Company LLC), SODIUM CHLORIDE (Medefil), SODIUM CHLORIDE (Medical Purchasing Solutions), SODIUM CHLORIDE (Medical Purchasing Solutions), SODIUM CHLORIDE (ProPharma Distribution), SODIUM CHLORIDE (Sportpharm), SODIUM CHLORIDE FOR IRRIGATION (B. Braun Medical) |
|---|
| Generic |
0.9% SODIUM CHLORIDE (HF Acquisition Co LLC), 0.9% SODIUM CHLORIDE (HF Acquisition Co LLC), 0.9% SODIUM CHLORIDE INJECTION, 500ML, DRE BIOLAB, TOTALCARE (AMCO INTERNATIONAL CORP), 3% SODIUM CHLORIDE (Becton Dickinson and Company), BACTERIOSTATIC SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Becton Dickinson and Company), SODIUM CHLORIDE (Becton Dickinson and Company), SODIUM CHLORIDE (Becton Dickinson and Company), SODIUM CHLORIDE (CODAN US CORPORATION), SODIUM CHLORIDE (Cardinal Health 107), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Kabi USA), SODIUM CHLORIDE (Fresenius Medical Care Renal Therapies Group), SODIUM CHLORIDE (Fresenius Medical Care de Mexico), SODIUM CHLORIDE (Henry Schein), SODIUM CHLORIDE (Henry Schein), SODIUM CHLORIDE (Hikma Pharmaceuticals USA), SODIUM CHLORIDE (LABORATORIOS GRIFOLS SA), SODIUM CHLORIDE (Nephron SC), SODIUM CHLORIDE (Nextgen Pharmaceuticals LLC), SODIUM CHLORIDE (Nexus Pharmaceuticals LLC), SODIUM CHLORIDE (SOLA Pharmaceuticals), SODIUM CHLORIDE (Spectra Medical Deviecs) |
|---|
| Formula |
Cl. Na
|
|---|
| Exact mass |
57.9586
|
|---|
| Mol weight |
58.44
|
|---|
| Structure |
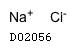
|
|---|
| Remark |
| Product (mixture): | D04109<JP> D04113<JP> D04114<JP> D04424<JP> D04981<JP> D05329<JP/US> D05352<JP> D07703<JP> D08810<JP> D08814<JP> D08816<JP> D11174<JP> D11552<US> |
|
|---|
| Efficacy |
Pharmaceutic aid (tonicity agent), Replenisher (electrolyte), Supplement (sodium chloride) |
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
A ALIMENTARY TRACT AND METABOLISM
A12 MINERAL SUPPLEMENTS
A12C OTHER MINERAL SUPPLEMENTS
A12CA Sodium
A12CA01 Sodium chloride
D02056 Sodium chloride (JP18/USP) <JP/US>
B BLOOD AND BLOOD FORMING ORGANS
B05 BLOOD SUBSTITUTES AND PERFUSION SOLUTIONS
B05C IRRIGATING SOLUTIONS
B05CB Salt solutions
B05CB01 Sodium chloride
D02056 Sodium chloride (JP18/USP) <JP/US>
B05X I.V. SOLUTION ADDITIVES
B05XA Electrolyte solutions
B05XA03 Sodium chloride
D02056 Sodium chloride (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Electrolytes/Minerals/Metals/Vitamins
Electrolyte/ Mineral Replacement
Sodium
D02056 Sodium chloride (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
3 Agents affecting metabolism
33 Blood and body fluid agents
331 Blood substitutes
3311 Physiological salines
D02056 Sodium chloride (JP18/USP)
3319 Others
D02056 Sodium chloride (JP18/USP)
7 Agents not mainly for therapeutic purpose
71 Dispensing medicines
719 Miscellaneous
7190 Miscellaneous
D02056 Sodium chloride (JP18/USP)
Classification of Japanese OTC drugs [BR:br08313]
Agents for ophthalmologic use
65 Artificial tear
D02056 Sodium chloride (JP18/USP)
Other (not belonging to any other category)
85 Other (not belonging to any other category)
D02056 Sodium chloride (JP18/USP)
Risk category of Japanese OTC drugs [BR:br08312]
Third-class OTC drugs
Inorganic and organic chemicals
Sodium chloride
D02056 Sodium chloride (JP18/USP)
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D02056 Sodium chloride
D02056 10% Sodium chloride injection
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 2
1 Z Na 25.6200 -18.1067 #+
2 X Cl 28.5133 -18.1067 #-
BOND 0
|
|---|
|
» Japanese version » Back
|