| Entry |
|
|---|
| Name |
Cyclizine hydrochloride (USP) |
|---|
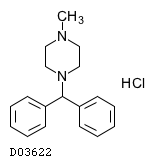
| Formula |
C18H22N2. HCl
|
|---|
| Exact mass |
302.1550
|
|---|
| Mol weight |
302.84
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
Anti-allergic agent
DG01557 Histamine receptor antagonist
DG01482 Histamine receptor H1 antagonist
|
|---|
| Remark |
|
|---|
| Efficacy |
Anti-emetic, H1 receptor antagonist |
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
| hsa04750 | Inflammatory mediator regulation of TRP channels |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07212 | Histamine H1 receptor antagonists |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
R RESPIRATORY SYSTEM
R06 ANTIHISTAMINES FOR SYSTEMIC USE
R06A ANTIHISTAMINES FOR SYSTEMIC USE
R06AE Piperazine derivatives
R06AE03 Cyclizine
D03622 Cyclizine hydrochloride (USP)
Drug groups [BR:br08330]
Anti-allergic agent
DG01557 Histamine receptor antagonist
DG01482 Histamine receptor H1 antagonist
DG01105 Cyclizine
D03622 Cyclizine hydrochloride
Target-based classification of drugs [BR:br08310]
G Protein-coupled receptors
Rhodopsin family
Histamine
HRH1
D03622 Cyclizine hydrochloride (USP)
Drug groups [BR:br08330]
Anti-allergic agent
DG01557 Histamine receptor antagonist
DG01482 Histamine receptor H1 antagonist
DG01105 Cyclizine
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 21
1 X Cl 29.2600 -15.7500
2 C8x C 20.3696 -17.3576
3 C8x C 20.3696 -18.7575
4 C8x C 21.5819 -19.4574
5 C8x C 22.7943 -18.7575
6 C8y C 22.7943 -17.3576
7 C8x C 21.5819 -16.6577
8 C8x C 25.2188 -18.7575
9 C8y C 25.2188 -17.3576
10 C1c C 24.0065 -16.6577
11 C8x C 26.4311 -19.4574
12 C8x C 27.6434 -18.7575
13 C8x C 27.6434 -17.3576
14 C8x C 26.4311 -16.6577
15 N1y N 24.0065 -15.2579
16 C1x C 25.2209 -14.5568
17 C1x C 25.2209 -13.1569
18 N1y N 24.0085 -12.4570
19 C1x C 22.7943 -13.1581
20 C1x C 22.7943 -14.5580
21 C1a C 24.0085 -11.0584
BOND 22
1 2 3 2
2 3 4 1
3 4 5 2
4 5 6 1
5 6 7 2
6 2 7 1
7 8 9 1
8 9 10 1
9 6 10 1
10 8 11 2
11 11 12 1
12 12 13 2
13 13 14 1
14 9 14 2
15 10 15 1
16 15 16 1
17 16 17 1
18 17 18 1
19 18 19 1
20 19 20 1
21 15 20 1
22 18 21 1
|
|---|