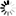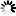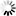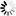Monobactams are beta-lactam antibiotics containing a monocyclic beta-lactam nucleus, which is structurally different from penicillin and cephalosporin core structures with another fused ring. This diagram shows biosynthesis of nocardicin A, a naturally occurring monobactam, via the pentapeptide formed by condensation of L-4-hydroxyphenylglycine (L-pHPG), L-arginine and L-serine [MD:
M00736]. Other naturally occurring monobactams are also shown but the biosynthetic pathway is not yet fully characterized. Sulfazecin and other 3-aminomonobactamic acid derivatives are derived from serine or threonine, and tabtoxinine-beta-lactam is a phytotoxin. Aztreonam, a synthetic monobactam originally isolated as SQ 26,180 from Chromobacterium violaceum, is the first clinically used monobactam.
 Monobactam biosynthesis - Sphingobium sp. YBL2
Monobactam biosynthesis - Sphingobium sp. YBL2
 Monobactam biosynthesis - Sphingobium sp. YBL2
Monobactam biosynthesis - Sphingobium sp. YBL2