| Entry |
|
|---|
| Name |
Montelukast sodium (JP18/USP);
Singulair (TN) |
|---|
| Product |
|
|---|
| Generic |
MONTELUKAST (A-S Medication Solutions), MONTELUKAST (A-S Medication Solutions), MONTELUKAST (Aphena Pharma Solutions - Tennessee), MONTELUKAST (AvPAK), MONTELUKAST (BluePoint Laboratories), MONTELUKAST (Bryant Ranch Prepack), MONTELUKAST (Legacy Pharmaceutical Packaging), MONTELUKAST (Macleods Pharmaceuticals Limited), MONTELUKAST (NuCare Pharmaceuticals), MONTELUKAST (NuCare Pharmaceuticals), MONTELUKAST (Preferred Pharmaceuticals), MONTELUKAST (Proficient Rx LP), MONTELUKAST (Quallent Pharmaceuticals Health LLC), MONTELUKAST (REMEDYREPACK), MONTELUKAST (XLCare Pharmaceuticals), MONTELUKAST SODIUM (A-S Medication Solutions), MONTELUKAST SODIUM (A-S Medication Solutions), MONTELUKAST SODIUM (Accord Healthcare), MONTELUKAST SODIUM (Ajanta Pharma USA), MONTELUKAST SODIUM (American Health Packaging), MONTELUKAST SODIUM (Amneal Pharmaceuticals LLC), MONTELUKAST SODIUM (Amneal Pharmaceuticals LLC), MONTELUKAST SODIUM (Aurobindo Pharma Limited), MONTELUKAST SODIUM (Aurobindo Pharma Limited), MONTELUKAST SODIUM (AvKARE), MONTELUKAST SODIUM (AvPAK), MONTELUKAST SODIUM (Bryant Ranch Prepack), MONTELUKAST SODIUM (Bryant Ranch Prepack), MONTELUKAST SODIUM (Bryant Ranch Prepack), MONTELUKAST SODIUM (Camber Pharmaceuticals), MONTELUKAST SODIUM (Camber Pharmaceuticals), MONTELUKAST SODIUM (Cardinal Health 107), MONTELUKAST SODIUM (Chartwell RX), MONTELUKAST SODIUM (Coupler LLC), MONTELUKAST SODIUM (Coupler LLC), MONTELUKAST SODIUM (DIRECT RX), MONTELUKAST SODIUM (Direct_Rx), MONTELUKAST SODIUM (Dr.Reddy's Laboratories Limited), MONTELUKAST SODIUM (Lannett Company), MONTELUKAST SODIUM (Macleods Pharmaceuticals Limited), MONTELUKAST SODIUM (Major Pharmaceuticals), MONTELUKAST SODIUM (NuCare Pharmaceuticals), MONTELUKAST SODIUM (NuCare Pharmaceuticals), MONTELUKAST SODIUM (NuCare Pharmaceuticals), MONTELUKAST SODIUM (PD-Rx Pharmaceuticals), MONTELUKAST SODIUM (PD-Rx Pharmaceuticals), MONTELUKAST SODIUM (PD-Rx Pharmaceuticals), MONTELUKAST SODIUM (Preferred Pharmaceuticals), MONTELUKAST SODIUM (Preferred Pharmaceuticals), MONTELUKAST SODIUM (Preferred Pharmaceuticals), MONTELUKAST SODIUM (Preferred Pharmaceuticals), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Proficient Rx LP), MONTELUKAST SODIUM (Redpharm Drug), MONTELUKAST SODIUM (Redpharm Drug), MONTELUKAST SODIUM (Redpharn Drug), MONTELUKAST SODIUM (Rising Pharma Holdings), MONTELUKAST SODIUM (Rising Pharma Holdings), MONTELUKAST SODIUM (Torrent Pharmaceuticals Limited), MONTELUKAST SODIUM (Unichem Pharmaceuticals (USA)), MONTELUKAST SODIUM (Westminster Pharmaceuticals) |
|---|
| Formula |
C35H35ClNO3S. Na
|
|---|
| Exact mass |
607.1924
|
|---|
| Mol weight |
608.16
|
|---|
| Structure |
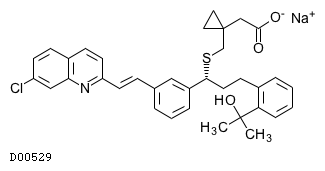
|
|---|
| Simcomp |
|
|---|
| Class |
Anti-allergic agent
DG01541 Cysteinyl leukotriene receptor antagonist
Metabolizing enzyme substrate
DG02918 CYP2C8 substrate
DG01642 CYP2C9 substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
|
|---|
| Remark |
| Therapeutic category: | 4490 |
| Product (DG01066): | D00529<JP/US> |
|
|---|
| Efficacy |
Antiallergic, Antiasthmatic, Leukotriene receptor antagonist |
|---|
| Disease |
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
|
|---|
| Metabolism |
Enzyme: CYP2C8 [HSA: 1558], CYP2C9 [HSA: 1559], CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07228 | Eicosanoid receptor agonists/antagonists |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
R RESPIRATORY SYSTEM
R03 DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
R03D OTHER SYSTEMIC DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
R03DC Leukotriene receptor antagonists
R03DC03 Montelukast
D00529 Montelukast sodium (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Respiratory Tract/Pulmonary Agents
Antileukotrienes
Montelukast
D00529 Montelukast sodium (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
4 Agents affecting cellular function
44 Allergic agents
449 Miscellaneous
4490 Miscellaneous
D00529 Montelukast sodium (JP18/USP)
Drug groups [BR:br08330]
Anti-allergic agent
DG01541 Cysteinyl leukotriene receptor antagonist
DG01066 Montelukast
D00529 Montelukast sodium
Metabolizing enzyme substrate
DG02918 CYP2C8 substrate
DG01066 Montelukast
D00529 Montelukast sodium
DG01642 CYP2C9 substrate
DG01066 Montelukast
D00529 Montelukast sodium
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG01066 Montelukast
D00529 Montelukast sodium
Target-based classification of drugs [BR:br08310]
G Protein-coupled receptors
Rhodopsin family
Cysteinyl leukotriene
CYSLTR1
D00529 Montelukast sodium (JP18/USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00529 Montelukast sodium
D00529 Montelukast sodium tablets
D00529 Montelukast sodium chewable tablets
D00529 Montelukast sodium granules
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D00529
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00529
Drug groups [BR:br08330]
Anti-allergic agent
DG01541 Cysteinyl leukotriene receptor antagonist
DG01066 Montelukast
Metabolizing enzyme substrate
DG02918 CYP2C8 substrate
DG01066 Montelukast
DG01642 CYP2C9 substrate
DG01066 Montelukast
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG01066 Montelukast
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 42
1 C1z C 36.9691 -14.8166
2 C1x C 36.2578 -13.6037
3 C1x C 35.5580 -14.8283
4 C1b C 36.9691 -16.2103
5 C1b C 38.1704 -14.1168
6 S2a S 35.7562 -16.9160
7 C1c C 35.7621 -18.3156
8 C8y C 34.5549 -19.0211
9 C1b C 36.9750 -19.0094
10 C8x C 33.3419 -18.3214
11 C8x C 34.5549 -20.4208
12 C1b C 38.1762 -18.3097
13 C8y C 32.1290 -19.0270
14 C8x C 33.3419 -21.1263
15 C8y C 39.3893 -19.0037
16 C2b C 30.9160 -18.3330
17 C8x C 32.1290 -20.4265
18 C8y C 39.3951 -20.3974
19 C8x C 40.5964 -18.3039
20 C2b C 29.7030 -19.0270
21 C8x C 40.6081 -21.0972
22 C1d C 38.1821 -21.1029
23 C8x C 41.8093 -18.9920
24 C8y C 28.5017 -18.3330
25 C8x C 41.8152 -20.3917
26 C1a C 36.9691 -21.7911
27 N5x N 27.2831 -19.0270
28 C8x C 28.5076 -16.9335
29 C8y C 26.1399 -18.3273
30 C8x C 27.2947 -16.2220
31 C8y C 26.0759 -16.9218
32 C8x C 24.8687 -19.0211
33 C8x C 24.8687 -16.2162
34 C8y C 23.6500 -18.3273
35 C8x C 23.6500 -16.9218
36 X Cl 22.4311 -19.0211
37 C6a C 39.3798 -14.8090
38 O6a O 40.5980 -14.0993 #-
39 O6a O 39.3846 -16.2398
40 C1a C 38.8570 -22.2784
41 O1a O 37.4629 -19.8502
42 Z Na 42.0700 -14.1400 #+
BOND 45
1 1 2 1
2 1 3 1
3 1 4 1
4 1 5 1
5 4 6 1
6 7 6 1 #Down
7 7 8 1
8 7 9 1
9 8 10 2
10 8 11 1
11 9 12 1
12 10 13 1
13 11 14 2
14 12 15 1
15 13 16 1
16 13 17 2
17 15 18 2
18 15 19 1
19 16 20 2
20 18 21 1
21 18 22 1
22 19 23 2
23 20 24 1
24 21 25 2
25 22 26 1
26 24 27 2
27 24 28 1
28 27 29 1
29 28 30 2
30 29 31 2
31 29 32 1
32 31 33 1
33 32 34 2
34 33 35 2
35 34 36 1
36 2 3 1
37 14 17 1
38 23 25 1
39 30 31 1
40 34 35 1
41 5 37 1
42 37 38 1
43 37 39 2
44 22 40 1
45 22 41 1
|
|---|
|