 | | DRUG: Clindamycin phosphate | |
| Entry |
|
|---|
| Name |
Clindamycin phosphate (JP18/USP);
Cleocin (TN);
Dalacin-S (TN);
Evoclin (TN) |
|---|
| Product |
CLEOCIN (Pharmacia & Upjohn Company LLC), CLEOCIN (Pharmacia & Upjohn Company LLC), CLEOCIN PHOSPHATE (Henry Schein), CLEOCIN PHOSPHATE (Medical Purchasing Solutions), CLEOCIN PHOSPHATE (Medical Purchasing Solutions), CLEOCIN PHOSPHATE (Medical Purchasing Solutions), CLEOCIN PHOSPHATE (Medical Purchasing Solutions), CLEOCIN PHOSPHATE (Pharmacia & Upjohn Company LLC), CLEOCIN PHOSPHATE (Pharmacia & Upjohn Company LLC), CLEOCIN PHOSPHATE (ProPharma Distribution), CLEOCIN T (Pharmacia & Upjohn Company LLC), CLINDAGEL (Bausch Health US), CLINDAMYCIN PHOSPHATE (Baxter Healthcare Company), CLINDESSE (Padagis Israel Pharmaceuticals), XACIATO (Organon LLC) |
|---|
| Generic |
CLINDACIN (Medimetriks Pharmaceuticals), CLINDACIN ETZ (Medimetriks Pharmaceuticals), CLINDACIN P (Medimetriks Pharmaceuticals), CLINDAMYCIN (Henry Schein), CLINDAMYCIN (Medical Purchasing Solutions), CLINDAMYCIN (Medical Purchasing Solutions), CLINDAMYCIN (Medical Purchasing Solutions), CLINDAMYCIN (Medical Purchasing Solutions), CLINDAMYCIN (Sagent Pharmaceuticals), CLINDAMYCIN IN 5 PERCENT DEXTROSE (Civica), CLINDAMYCIN IN 5 PERCENT DEXTROSE (Sandoz), CLINDAMYCIN PHOAPHATE TOPICAL SOLUTION USP, 1% (Encube Ethicals Private Limited), CLINDAMYCIN PHOAPHATE TOPICAL SOLUTION USP, 1% (Northstar Rx LLC), CLINDAMYCIN PHOAPHATE TOPICAL SOLUTION, 1% (Preferred Pharmaceuticals), CLINDAMYCIN PHOAPHATE TOPICAL USP, 1% (A-S Medication Solutions), CLINDAMYCIN PHOSPHATE (A-S Medication Solutions), CLINDAMYCIN PHOSPHATE (A-S Medication Solutions), CLINDAMYCIN PHOSPHATE (A-S Medication Solutions), CLINDAMYCIN PHOSPHATE (A-S Medication Solutions), CLINDAMYCIN PHOSPHATE (A-S Medication Solutions), CLINDAMYCIN PHOSPHATE (A-S Medication Solutions), CLINDAMYCIN PHOSPHATE (Alembic Pharmaceuticals Limited), CLINDAMYCIN PHOSPHATE (Alembic Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Amneal Pharmaceuticals NY LLC), CLINDAMYCIN PHOSPHATE (Amneal Pharmaceuticals NY LLC), CLINDAMYCIN PHOSPHATE (Baxter Healthcare Company), CLINDAMYCIN PHOSPHATE (Baxter Healthcare Company), CLINDAMYCIN PHOSPHATE (Baxter Healthcare Corporation), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Bryant Ranch Prepack), CLINDAMYCIN PHOSPHATE (Carnegie Pharmaceuticals), CLINDAMYCIN PHOSPHATE (E. Fougera & Co. a division of Fougera Pharmaceuticals), CLINDAMYCIN PHOSPHATE (E. Fougera & Co. a division of Fougera Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Encube Ethicals Private Limited), CLINDAMYCIN PHOSPHATE (Encube Ethicals Private Limited), CLINDAMYCIN PHOSPHATE (GLENMARK PHARMACEUTICALS), CLINDAMYCIN PHOSPHATE (GLENMARK PHARMACEUTICALS), CLINDAMYCIN PHOSPHATE (Glasshouse Pharmaceuticals Limited Canada), CLINDAMYCIN PHOSPHATE (Lifsa Drugs LLC), CLINDAMYCIN PHOSPHATE (Mylan Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Mylan Pharmaceuticals), CLINDAMYCIN PHOSPHATE (NORTHSTAR RX LLC), CLINDAMYCIN PHOSPHATE (NORTHSTAR RX LLC), CLINDAMYCIN PHOSPHATE (Oceanside Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Padagis Israel Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Padagis Israel Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Padagis Israel Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Padagis Israel Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Padagis Israel Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Preferred Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Preferred Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Solaris Pharma Corporation), CLINDAMYCIN PHOSPHATE (Solaris Pharma Corporation), CLINDAMYCIN PHOSPHATE (Sun Pharmaceutical Industries), CLINDAMYCIN PHOSPHATE (Sun Pharmaceutical Industries), CLINDAMYCIN PHOSPHATE (Taro Pharmaceuticals U.S.A.), CLINDAMYCIN PHOSPHATE (Taro Pharmaceuticals U.S.A.), CLINDAMYCIN PHOSPHATE (Viona Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Viona Pharmaceuticals), CLINDAMYCIN PHOSPHATE (Zydus Lifesciences Limited), CLINDAMYCIN PHOSPHATE (Zydus Lifesciences Limited), CLINDAMYCIN PHOSPHATE (i3 Pharmaceuticals) |
|---|
| Formula |
C18H34ClN2O8PS
|
|---|
| Exact mass |
504.1462
|
|---|
| Mol weight |
504.96
|
|---|
| Structure |
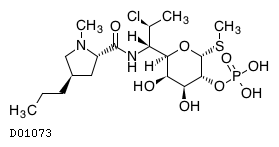
|
|---|
| Simcomp |
|
|---|
| Class |
Antibacterial
DG01578 Lincosamide antibiotic
|
|---|
| Remark |
| Product (DG00435): | D00277<US> D01073<JP/US> D01990<US> D02132<JP/US> |
| Product (mixture): | D10602<JP/US> D11084<US> D12969<US> |
|
|---|
| Efficacy |
Antibacterial, Protein biosynthesis inhibitor |
|---|
| Disease |
Infections due to susceptible strains of Streptococci [DS: H00333] Acne vulgaris [DS: H01445] |
|---|
| Comment |
Semisynthetic lincosamide
Active form of prodrug: Clindamycin [DR: D00277]
|
|---|
| Target |
50S ribosomal subunit |
|---|
| Pathway |
|
|---|
| Interaction |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
D DERMATOLOGICALS
D10 ANTI-ACNE PREPARATIONS
D10A ANTI-ACNE PREPARATIONS FOR TOPICAL USE
D10AF Antiinfectives for treatment of acne
D10AF01 Clindamycin
D01073 Clindamycin phosphate (JP18/USP) <JP/US>
G GENITO URINARY SYSTEM AND SEX HORMONES
G01 GYNECOLOGICAL ANTIINFECTIVES AND ANTISEPTICS
G01A ANTIINFECTIVES AND ANTISEPTICS, EXCL. COMBINATIONS WITH CORTICOSTEROIDS
G01AA Antibiotics
G01AA10 Clindamycin
D01073 Clindamycin phosphate (JP18/USP) <JP/US>
J ANTIINFECTIVES FOR SYSTEMIC USE
J01 ANTIBACTERIALS FOR SYSTEMIC USE
J01F MACROLIDES, LINCOSAMIDES AND STREPTOGRAMINS
J01FF Lincosamides
J01FF01 Clindamycin
D01073 Clindamycin phosphate (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Antibacterials
Antibacterials, Other
Clindamycin
D01073 Clindamycin phosphate (JP18/USP)
Dermatological Agents
Topical Anti-infectives
Antibacterials, Dermatological
Clindamycin
D01073 Clindamycin phosphate (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
26 Epidermides
263 Suppurative dermatosis agents
2634 Antibiotics for external use
D01073 Clindamycin phosphate (JP18/USP)
6 Agents against pathologic organisms and parasites
61 Antibiotics
611 Acting mainly on gram-positive bacteria
6112 Lincomycins
D01073 Clindamycin phosphate (JP18/USP)
Drug groups [BR:br08330]
Antibacterial
DG01578 Lincosamide antibiotic
DG00435 Clindamycin
D01073 Clindamycin phosphate
Drug classes [BR:br08332]
Antibacterial
DG01578 Lincosamide antibiotic
D01073 Clindamycin phosphate
Antimicrobials [BR:br08307]
Antibacterials
Protein biosynthesis inhibitor
Lincosamide
D01073 Clindamycin phosphate (JP18/USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D01073 Clindamycin phosphate
D01073 Clindamycin phosphate injection
Prodrugs [br08324.html]
D01073
Drug groups [BR:br08330]
Antibacterial
DG01578 Lincosamide antibiotic
DG00435 Clindamycin
Antimicrobials abbreviations [BR:br08327]
Antibacterials
Protein biosynthesis inhibitor
Lincosamide
DG00435 Clindamycin
Prodrugs [br08324.html]
DG00435
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 31
1 C1y C 20.3420 -15.6751
2 C1y C 20.3420 -17.0766
3 C1y C 21.5567 -17.7773
4 C1y C 22.7713 -17.0766
5 C1y C 22.7713 -15.6751
6 O2x O 21.5567 -14.9744
7 S2a S 23.9859 -14.9744
8 C1a C 23.9859 -13.5730
9 O2b O 23.9859 -17.7773
10 O1a O 21.5567 -19.1789
11 O1a O 19.1275 -17.7773
12 C1c C 19.1275 -14.9744
13 N1b N 17.9129 -15.6751
14 C1c C 19.1275 -13.5730
15 X Cl 17.9129 -12.8722
16 C1a C 20.3420 -12.8722
17 C5a C 16.6983 -14.9744
18 C1y C 15.4837 -15.6751
19 O5a O 16.6983 -13.5730
20 N1y N 14.3578 -14.8803
21 C1x C 13.2387 -15.7030
22 C1y C 13.6855 -17.0322
23 C1x C 15.0690 -17.0253
24 C1a C 14.3578 -13.4808
25 C1b C 12.9918 -18.2479
26 C1b C 11.6141 -18.2550
27 C1a C 10.9162 -19.4785
28 P1b P 25.2093 -17.0704
29 O1c O 26.4215 -16.3706
30 O1c O 24.5068 -15.8540
31 O1c O 25.9066 -18.2777
BOND 32
1 1 2 1
2 2 3 1
3 3 4 1
4 4 5 1
5 5 6 1
6 1 6 1
7 5 7 1 #Down
8 7 8 1
9 4 9 1 #Down
10 3 10 1 #Up
11 2 11 1 #Up
12 1 12 1
13 12 13 1
14 12 14 1
15 14 15 1 #Up
16 14 16 1
17 13 17 1
18 18 17 1 #Down
19 17 19 2
20 18 20 1
21 20 21 1
22 21 22 1
23 22 23 1
24 18 23 1
25 20 24 1
26 22 25 1 #Up
27 25 26 1
28 26 27 1
29 9 28 1
30 28 29 1
31 28 30 2
32 28 31 1
|
|---|
|
» Japanese version » Back
|