 | | DRUG: Sumatriptan succinate | |
| Entry |
|
|---|
| Name |
Sumatriptan succinate (JAN/USP);
Alsuma (TN);
Imitrex (TN);
Sumave dosepro (TN);
Zecutity (TN) |
|---|
| Product |
|
|---|
| Generic |
SUMATRIPTAN (A-S Medication Solutions), SUMATRIPTAN (A-S Medication Solutions), SUMATRIPTAN (A-S Medication Solutions), SUMATRIPTAN (Advanced Rx Pharmacy of Tennessee), SUMATRIPTAN (Advanced Rx Pharmacy of Tennessee), SUMATRIPTAN (Advanced Rx of Tennessee), SUMATRIPTAN (Advanced Rx of Tennessee), SUMATRIPTAN (Asclemed USA), SUMATRIPTAN (Aurobindo Pharma Limited), SUMATRIPTAN (Baxter Healthcare Corporation), SUMATRIPTAN (Bionpharma), SUMATRIPTAN (Caplin Steriles Limited), SUMATRIPTAN (Direct_Rx), SUMATRIPTAN (Eugia US LLC), SUMATRIPTAN (Eugia US LLC), SUMATRIPTAN (Golden State Medical Supply), SUMATRIPTAN (Hikma Pharmaceuticals USA), SUMATRIPTAN (NorthStar Rx LLC), SUMATRIPTAN (NuCare Pharmaceuticals), SUMATRIPTAN (NuCare Pharmaceuticals), SUMATRIPTAN (NuCare Pharmaceuticals), SUMATRIPTAN (NuCare Pharmaceuticals), SUMATRIPTAN (NuCare Pharmaceuticals), SUMATRIPTAN (NuCare Pharmaceuticals), SUMATRIPTAN (Prasco Laboratories), SUMATRIPTAN (Preferred Pharmaceuticals), SUMATRIPTAN (Preferred Pharmaceuticals), SUMATRIPTAN (Preferred Pharmaceuticals), SUMATRIPTAN (Preferred Pharmaceuticals), SUMATRIPTAN (Preferred Pharmaceuticals), SUMATRIPTAN (Proficient Rx LP), SUMATRIPTAN (Proficient Rx LP), SUMATRIPTAN (Proficient Rx LP), SUMATRIPTAN (Proficient Rx LP), SUMATRIPTAN (Proficient Rx LP), SUMATRIPTAN (Proficient Rx LP), SUMATRIPTAN (Quality Care Products), SUMATRIPTAN (Quality Care Products), SUMATRIPTAN (REMEDYREPACK), SUMATRIPTAN (REMEDYREPACK), SUMATRIPTAN (RedPharm Drug), SUMATRIPTAN (RedPharm Drug), SUMATRIPTAN (RedPharm Drug), SUMATRIPTAN (Rising Pharma Holdings), SUMATRIPTAN (SUN PHARMACEUTICAL INDUSTRIES), SUMATRIPTAN (Somerset Therapeutics), SUMATRIPTAN (Xellia Pharmaceuticals USA LLC), SUMATRIPTAN SUCCINATE (A-S Medication Solutions), SUMATRIPTAN SUCCINATE (Asclemed USA), SUMATRIPTAN SUCCINATE (Direct_Rx), SUMATRIPTAN SUCCINATE (Direct_Rx), SUMATRIPTAN SUCCINATE (Direct_Rx), SUMATRIPTAN SUCCINATE (Dr. Reddy's Laboratories Inc.,), SUMATRIPTAN SUCCINATE (Dr. Reddy's Laboratories Limited), SUMATRIPTAN SUCCINATE (Dr. Reddy's Laboratories Limited), SUMATRIPTAN SUCCINATE (Fresenius Kabi USA), SUMATRIPTAN SUCCINATE (Golden State Medical Supply), SUMATRIPTAN SUCCINATE (Mylan Pharmaceuticals), SUMATRIPTAN SUCCINATE (NorthStar Rx LLC), SUMATRIPTAN SUCCINATE (NuCare Pharmaceuticals), SUMATRIPTAN SUCCINATE (NuCare Pharmaceuticals), SUMATRIPTAN SUCCINATE (Preferred Pharmaceuticals), SUMATRIPTAN SUCCINATE (Proficient Rx LP), SUMATRIPTAN SUCCINATE (Proficient Rx LP), SUMATRIPTAN SUCCINATE (Proficient Rx LP), SUMATRIPTAN SUCCINATE (Proficient Rx LP), SUMATRIPTAN SUCCINATE (Proficient Rx LP), SUMATRIPTAN SUCCINATE (Proficient Rx LP), SUMATRIPTAN SUCCINATE (Proficient Rx LP), SUMATRIPTAN SUCCINATE (Quality Care Products), SUMATRIPTAN SUCCINATE (Sun Pharmaceutical Industries), SUMATRIPTAN SUCCINATE (Sun Pharmaceutical Industries), SUMATRIPTAN SUCCINATE (Wockhardt USA LLC.) |
|---|
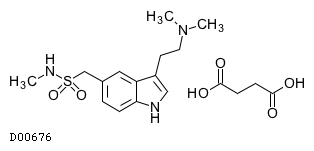
| Formula |
C14H21N3O2S. C4H6O4
|
|---|
| Exact mass |
413.1621
|
|---|
| Mol weight |
413.49
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
|
|---|
| Remark |
| Therapeutic category: | 2160 |
| Product (DG00836): | D00451<JP/US> D00676<JP/US> |
| Product (mixture): | D11577<US> |
|
|---|
| Efficacy |
Antimigraine, Vasoconstrictor, Serotonin receptor agonist |
|---|
| Disease |
|
|---|
| Comment |
Triptans
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
|
|---|
| Metabolism |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07211 | Serotonin receptor agonists/antagonists |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
N NERVOUS SYSTEM
N02 ANALGESICS
N02C ANTIMIGRAINE PREPARATIONS
N02CC Selective serotonin (5HT1) agonists
N02CC01 Sumatriptan
D00676 Sumatriptan succinate (JAN/USP) <JP/US>
USP drug classification [BR:br08302]
Antimigraine Agents
Serotonin (5-HT) Receptor Agonists
Sumatriptan
D00676 Sumatriptan succinate (JAN/USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
21 Cardiovascular agents
216 Vasoconstrictors
2160 Vasoconstrictors
D00676 Sumatriptan succinate (JAN/USP)
Drug groups [BR:br08330]
Analgesic
DG01484 5-HT1B-receptor agonist
DG00836 Sumatriptan
D00676 Sumatriptan succinate
DG01485 5-HT1D-receptor agonist
DG00836 Sumatriptan
D00676 Sumatriptan succinate
DG01518 5-HT1B/1D-receptor agonist
DG00836 Sumatriptan
D00676 Sumatriptan succinate
DG01943 Triptan
DG00836 Sumatriptan
D00676 Sumatriptan succinate
Metabolizing enzyme substrate
DG02917 MAO substrate
DG03178 MAOA substrate
DG00836 Sumatriptan
D00676 Sumatriptan succinate
Target-based classification of drugs [BR:br08310]
G Protein-coupled receptors
Rhodopsin family
Serotonin
HTR1B
D00676 Sumatriptan succinate (JAN/USP) <JP/US>
HTR1D
D00676 Sumatriptan succinate (JAN/USP) <JP/US>
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D00676
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00676
Drug groups [BR:br08330]
Analgesic
DG01484 5-HT1B-receptor agonist
DG00836 Sumatriptan
DG01485 5-HT1D-receptor agonist
DG00836 Sumatriptan
DG01518 5-HT1B/1D-receptor agonist
DG00836 Sumatriptan
DG01943 Triptan
DG00836 Sumatriptan
Metabolizing enzyme substrate
DG02917 MAO substrate
DG03178 MAOA substrate
DG00836 Sumatriptan
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 28
1 C8y C 23.6794 -17.9951
2 C8y C 23.6853 -19.3996
3 C8y C 25.0140 -17.5639
4 C8x C 22.4731 -17.2843
5 N4x N 25.0197 -19.8250
6 C8x C 22.4673 -20.0930
7 C8x C 25.8357 -18.6886
8 C1b C 25.3287 -16.1944
9 C8y C 21.2553 -17.9893
10 C8x C 21.2610 -19.3937
11 C1b C 26.6633 -15.7807
12 C1b C 20.0373 -17.2957
13 N1c N 26.9720 -14.4170
14 S4a S 18.8310 -18.0010
15 C1a C 28.3066 -14.0033
16 C1a C 25.9464 -13.4671
17 N1b N 17.6247 -17.3017
18 O3c O 19.8217 -19.0033
19 O3c O 17.8344 -19.0033
20 C1a C 16.4124 -18.0067
21 C1b C 30.7583 -18.8477
22 C1b C 31.9646 -18.1484
23 C6a C 29.5461 -18.1543
24 C6a C 33.1767 -18.8420
25 O6a O 28.3397 -18.8536
26 O6a O 29.5461 -16.7557
27 O6a O 34.3889 -18.1426
28 O6a O 33.1767 -20.2406
BOND 28
1 1 2 1
2 1 3 1
3 1 4 2
4 2 5 1
5 2 6 2
6 3 7 2
7 3 8 1
8 4 9 1
9 6 10 1
10 8 11 1
11 9 12 1
12 11 13 1
13 12 14 1
14 13 15 1
15 13 16 1
16 14 17 1
17 14 18 2
18 14 19 2
19 17 20 1
20 5 7 1
21 9 10 2
22 21 22 1
23 21 23 1
24 22 24 1
25 23 25 1
26 23 26 2
27 24 27 1
28 24 28 2
|
|---|
|
» Japanese version » Back
|