| Entry |
|
|---|
| Name |
Valsartan (JP18/USP/INN);
Diovan (TN) |
|---|
| Product |
|
|---|
| Generic |
VALSARTAN (A-S Medication Solutions), VALSARTAN (A-S Medication Solutions), VALSARTAN (A-S Medication Solutions), VALSARTAN (A-S Medication Solutions), VALSARTAN (A-S Medication Solutions), VALSARTAN (A-S Medication Solutions), VALSARTAN (A-S Medication Solutions), VALSARTAN (A-S Medication Solutions), VALSARTAN (ANI Pharmaceuticals), VALSARTAN (Advagen Pharma), VALSARTAN (Alembic Pharmaceuticals Limited), VALSARTAN (Alembic Pharmaceuticals), VALSARTAN (American Health Packaging), VALSARTAN (Amneal Pharmaceuticals LLC), VALSARTAN (Amneal Pharmaceuticals of New York LLC), VALSARTAN (Aphena Pharma Solutions - Tennessee), VALSARTAN (Archis Pharma LLC), VALSARTAN (Ascend Laboratories), VALSARTAN (Aurobindo Pharma Limited), VALSARTAN (AvKARE), VALSARTAN (AvPAK), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Bryant Ranch Prepack), VALSARTAN (Camber Pharmaceuticals), VALSARTAN (Camber Pharmaceuticals), VALSARTAN (Coupler LLC), VALSARTAN (Coupler LLC), VALSARTAN (Direct_Rx), VALSARTAN (Direct_Rx), VALSARTAN (Dr. Reddy's Laboratories Limited), VALSARTAN (Jubilant Cadista Pharmacuticals), VALSARTAN (Lifsa Drugs LLC), VALSARTAN (Lupin Pharmaceuticals), VALSARTAN (Macleods Pharmaceuticals Limited), VALSARTAN (Mylan Pharmaceuticals), VALSARTAN (Northwind Pharmaceuticals), VALSARTAN (Northwind Pharmaceuticals), VALSARTAN (Northwind Pharmaceuticals), VALSARTAN (Northwind Pharmaceuticals), VALSARTAN (Northwind Pharmaceuticals), VALSARTAN (Northwind Pharmaceuticals), VALSARTAN (Northwind Pharmaceuticals), VALSARTAN (Ohm Laboratories), VALSARTAN (PD-Rx Pharmaceuticals), VALSARTAN (Proficient Rx LP), VALSARTAN (Proficient Rx LP), VALSARTAN (Proficient Rx LP), VALSARTAN (SQUARE PHARMACEUTICALS LIMITED), VALSARTAN (ScieGen Pharmaceuticals), VALSARTAN (Solco Healthcare US), VALSARTAN (Unichem Pharmaceuticals (USA)), VALSARTAN (Viona Pharmaceuticals), VALSARTAN (Zydus Lifesciences Limited) |
|---|
| Formula |
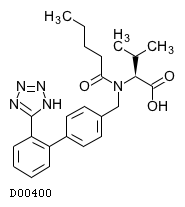
C24H29N5O3
|
|---|
| Exact mass |
435.2270
|
|---|
| Mol weight |
435.52
|
|---|
| Structure |
|
|---|
| Class |
Cardiovascular agent
DG01925 Renin-angiotensin system inhibitor
DG01495 Angiotensin II receptor antagonist
DG03231 Antihypertensive
DG01495 Angiotensin II receptor antagonist
Metabolizing enzyme substrate
DG01642 CYP2C9 substrate
Transporter substrate
DG02861 ABCC2 substrate
DG02856 SLCO1B1 substrate
DG02932 SLCO1B3 substrate
|
|---|
| Remark |
| Therapeutic category: | 2149 |
| Product (mixture): | D09197<JP/US> D09745<JP/US> D10286<US> D10525<JP> |
|
|---|
| Efficacy |
Antihypertensive, Angiotensin II receptor antagonist |
|---|
| Disease |
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
| hsa04261 | Adrenergic signaling in cardiomyocytes |
| hsa04270 | Vascular smooth muscle contraction |
|
|---|
| Metabolism |
Enzyme: CYP2C9 [HSA: 1559]
Transporter: SLCO1B1 [HSA: 10599], SLCO1B3 [HSA: 28234], ABCC2 [HSA: 1244]
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07229 | Angiotensin receptor and endothelin receptor antagonists |
|
|---|
| Other map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
C CARDIOVASCULAR SYSTEM
C09 AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
C09C ANGIOTENSIN II RECEPTOR BLOCKERS (ARBs), PLAIN
C09CA Angiotensin II receptor blockers (ARBs), plain
C09CA03 Valsartan
D00400 Valsartan (JP18/USP/INN) <JP/US>
USP drug classification [BR:br08302]
Cardiovascular Agents
Angiotensin II Receptor Antagonists
Valsartan
D00400 Valsartan (JP18/USP/INN)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
21 Cardiovascular agents
214 Antihypertensives
2149 Others
D00400 Valsartan (JP18/USP/INN)
Drug groups [BR:br08330]
Cardiovascular agent
DG01925 Renin-angiotensin system inhibitor
DG01495 Angiotensin II receptor antagonist
D00400 Valsartan
DG03231 Antihypertensive
DG01495 Angiotensin II receptor antagonist
D00400 Valsartan
Metabolizing enzyme substrate
DG01642 CYP2C9 substrate
D00400 Valsartan
Transporter substrate
DG02861 ABCC2 substrate
D00400 Valsartan
DG02856 SLCO1B1 substrate
D00400 Valsartan
DG02932 SLCO1B3 substrate
D00400 Valsartan
Drug classes [BR:br08332]
Cardiovascular agent
DG01495 Angiotensin II receptor antagonist
D00400 Valsartan
DG03231 Antihypertensive
D00400 Valsartan
Target-based classification of drugs [BR:br08310]
G Protein-coupled receptors
Rhodopsin family
Angiotensin
AGTR1
D00400 Valsartan (JP18/USP/INN) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00400 Valsartan
D00400 Valsartan tablets
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D00400
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00400
Drug transporters
D00400
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 32
1 C8x C 19.4226 -22.2154
2 C8x C 19.4226 -23.6234
3 C8x C 20.6194 -24.3274
4 C8x C 21.8865 -23.6234
5 C8y C 21.8865 -22.2154
6 C8y C 20.6194 -21.5115
7 C8y C 23.0833 -21.5115
8 C8x C 24.2801 -22.2154
9 C8x C 25.5472 -21.5115
10 C8y C 25.5472 -20.1035
11 C8x C 24.3504 -19.3995
12 C8x C 23.0833 -20.1035
13 C8y C 20.6194 -20.1035
14 N4x N 21.7457 -19.2587
15 N5x N 21.3233 -17.9916
16 N5x N 19.9154 -17.9916
17 N5x N 19.4930 -19.2587
18 C1b C 26.7440 -19.3995
19 N1c N 26.7440 -17.9916
20 C1c C 27.9407 -17.2876
21 C5a C 25.5472 -17.2876
22 O5a O 24.3504 -17.9916
23 C1b C 25.5472 -15.8797
24 C1b C 24.3504 -15.1757
25 C1b C 24.3504 -13.7677
26 C1a C 23.1537 -13.0637
27 C6a C 29.1375 -17.9916
28 O6a O 30.3343 -17.2876
29 O6a O 29.1375 -19.3995
30 C1c C 27.9407 -15.8797
31 C1a C 26.7440 -15.1757
32 C1a C 29.1375 -15.1757
BOND 34
1 1 2 2
2 2 3 1
3 3 4 2
4 4 5 1
5 5 6 2
6 1 6 1
7 5 7 1
8 7 8 2
9 8 9 1
10 9 10 2
11 10 11 1
12 11 12 2
13 7 12 1
14 6 13 1
15 13 14 1
16 14 15 1
17 15 16 2
18 16 17 1
19 13 17 2
20 10 18 1
21 18 19 1
22 19 20 1
23 19 21 1
24 21 22 2
25 21 23 1
26 23 24 1
27 24 25 1
28 25 26 1
29 20 27 1
30 27 28 2
31 27 29 1
32 20 30 1 #Up
33 30 31 1
34 30 32 1
|
|---|
|