 | | DRUG: Valacyclovir hydrochloride | |
| Entry |
|
|---|
| Name |
Valacyclovir hydrochloride (USP);
Valaciclovir hydrochloride (JP18);
Valtrex (TN) |
|---|
| Product |
|
|---|
| Generic |
VALACYCLOVIR (A-S Medication Solutions), VALACYCLOVIR (A-S Medication Solutions), VALACYCLOVIR (A-S Medication Solutions), VALACYCLOVIR (A-S Medication Solutions), VALACYCLOVIR (Bryant Ranch Prepack), VALACYCLOVIR (Bryant Ranch Prepack), VALACYCLOVIR (Camber Pharmaceuticals), VALACYCLOVIR (Chartwell RX), VALACYCLOVIR (Direct Rx), VALACYCLOVIR (Direct _Rx), VALACYCLOVIR (Direct_Rx), VALACYCLOVIR (Golden State Medical Supply), VALACYCLOVIR (McKesson Corporation dba SKY Packaging), VALACYCLOVIR (NuCare Pharmaceuticals), VALACYCLOVIR (NuCare Pharmaceuticals), VALACYCLOVIR (NuCare Pharmaceuticals), VALACYCLOVIR (NuCare Pharmaceuticals), VALACYCLOVIR (NuCare Pharmaceuticals), VALACYCLOVIR (PD-Rx Pharmaceuticals), VALACYCLOVIR (Preferred Pharmaceuticals), VALACYCLOVIR (Preferred Pharmaceuticals), VALACYCLOVIR (Proficient Rx LP), VALACYCLOVIR (Quallent Pharmaceuticals Health LLC), VALACYCLOVIR (REMEDYREPACK), VALACYCLOVIR (REMEDYREPACK), VALACYCLOVIR (Redpharm Drug), VALACYCLOVIR (SUN PHARMACEUTICAL INDUSTRIES), VALACYCLOVIR (St. Mary's Medical Park Pharmacy), VALACYCLOVIR (Westminster Pharmaceuticals), VALACYCLOVIR (Yiling Pharmaceutical), VALACYCLOVIR HYDROCHLORIDE (A-S Medication Solutions), VALACYCLOVIR HYDROCHLORIDE (A-S Medication Solutions), VALACYCLOVIR HYDROCHLORIDE (A-S Medication Solutions), VALACYCLOVIR HYDROCHLORIDE (A-S Medication Solutions), VALACYCLOVIR HYDROCHLORIDE (ASCLEMED USA), VALACYCLOVIR HYDROCHLORIDE (American Health Packaging), VALACYCLOVIR HYDROCHLORIDE (Asclemed USA), VALACYCLOVIR HYDROCHLORIDE (Asclemed USA), VALACYCLOVIR HYDROCHLORIDE (Aurobindo Pharma Limited), VALACYCLOVIR HYDROCHLORIDE (AvPAK), VALACYCLOVIR HYDROCHLORIDE (Bryant Ranch Prepack), VALACYCLOVIR HYDROCHLORIDE (Bryant Ranch Prepack), VALACYCLOVIR HYDROCHLORIDE (Bryant Ranch Prepack), VALACYCLOVIR HYDROCHLORIDE (Bryant Ranch Prepack), VALACYCLOVIR HYDROCHLORIDE (Cardinal Health 107), VALACYCLOVIR HYDROCHLORIDE (Cipla USA), VALACYCLOVIR HYDROCHLORIDE (Jubilant Cadista Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Major Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Mylan Institutional), VALACYCLOVIR HYDROCHLORIDE (Mylan Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NorthStar Rx LLC), VALACYCLOVIR HYDROCHLORIDE (Northwind Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Northwind Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Northwind Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NuCare Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NuCare Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NuCare Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NuCare Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NuCare Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NuCare Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (NuCare Pharmacuticals), VALACYCLOVIR HYDROCHLORIDE (PD-Rx Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (PD-Rx Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (PD-Rx Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (PD-Rx Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Preferred Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Preferred Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Preferred Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Preferred Pharmaceuticals), VALACYCLOVIR HYDROCHLORIDE (Proficient Rx LP), VALACYCLOVIR HYDROCHLORIDE (Proficient Rx LP), VALACYCLOVIR HYDROCHLORIDE (Proficient Rx LP), VALACYCLOVIR HYDROCHLORIDE (Proficient Rx LP), VALACYCLOVIR HYDROCHLORIDE (Proficient Rx LP), VALACYCLOVIR HYDROCHLORIDE (REMEDYREPACK), VALACYCLOVIR HYDROCHLORIDE (REMEDYREPACK), VALACYCLOVIR HYDROCHLORIDE (REMEDYREPACK), VALACYCLOVIR HYDROCHLORIDE (RedPharm Drug), VALACYCLOVIR HYDROCHLORIDE (RedPharm Drug), VALACYCLOVIR HYDROCHLORIDE (Redpharm Drug), VALACYCLOVIR HYDROCHLORIDE (Redpharm Drug), VALACYCLOVIR HYDROCHLORIDE (Rising Pharma Holdings), VALACYCLOVIR HYDROCHLORIDE (ST. MARY'S MEDICAL PARK PHARMACY), VALACYCLOVIR HYDROCHLORIDE (Sportpharm), VALACYCLOVIR HYDROCHLORIDE (Sportpharm), VALACYCLOVIR HYDROCHLORIDE (TIME CAP LABORATORIES) |
|---|
| Formula |
C13H20N6O4. HCl
|
|---|
| Exact mass |
360.1313
|
|---|
| Mol weight |
360.80
|
|---|
| Structure |
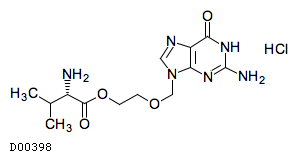
|
|---|
| Simcomp |
|
|---|
| Class |
Antiviral
DG02840 Anti-herpesvirus agent
|
|---|
| Remark |
| Therapeutic category: | 6250 |
| Product (DG00647): | D00398<JP/US> D10518<JP/US> |
|
|---|
| Efficacy |
Antiviral, DNA polymerase inhibitor |
|---|
| Disease |
|
|---|
| Comment |
Active form of prodrug: Acyclovir [DR: D00222]
|
|---|
| Target |
Herpesvirus DNA polymerase [KO: K18964] |
|---|
| Pathway |
|
|---|
| Interaction |
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
J ANTIINFECTIVES FOR SYSTEMIC USE
J05 ANTIVIRALS FOR SYSTEMIC USE
J05A DIRECT ACTING ANTIVIRALS
J05AB Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB11 Valaciclovir
D00398 Valacyclovir hydrochloride (USP) <JP/US>
USP drug classification [BR:br08302]
Antivirals
Antiherpetic Agents
Valacyclovir
D00398 Valacyclovir hydrochloride (USP)
Therapeutic category of drugs in Japan [BR:br08301]
6 Agents against pathologic organisms and parasites
62 Chemotherapeutics
625 Antivirals
6250 Antivirals
D00398 Valacyclovir hydrochloride (USP); Valaciclovir hydrochloride (JP18)
Drug groups [BR:br08330]
Antiviral
DG02840 Anti-herpesvirus agent
DG00647 Valaciclovir
D00398 Valacyclovir hydrochloride
Drug classes [BR:br08332]
Antiviral
DG02840 Anti-herpesvirus agent
D00398 Valacyclovir hydrochloride
Antimicrobials [BR:br08307]
Antivirals
Genome replication inhibitor
Herpesvirus DNA polymerase inhibitor
D00398 Valacyclovir hydrochloride (USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00398 Valaciclovir hydrochloride
D00398 Valaciclovir hydrochloride tablets
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D00398
Drug groups [BR:br08330]
Antiviral
DG02840 Anti-herpesvirus agent
DG00647 Valaciclovir
Antimicrobials abbreviations [BR:br08327]
Antivirals
Genome replication inhibitor
Herpesvirus DNA polymerase inhibitor
DG00647 Valaciclovir
Prodrugs [br08324.html]
DG00647
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 24
1 X Cl 33.8079 -15.4658
2 N4y N 26.2500 -17.2200
3 C8y C 27.5100 -16.7300
4 C8y C 27.5100 -15.3300
5 N5x N 26.1800 -14.9100
6 C8x C 25.4100 -16.1000
7 N5x N 28.7700 -17.4300
8 C8y C 29.9600 -16.7300
9 N4x N 29.9600 -15.3300
10 C8y C 28.7000 -14.5600
11 O5x O 28.7000 -13.1600
12 N1a N 31.2200 -17.3600
13 C1b C 26.2500 -18.6200
14 O2a O 24.9900 -19.3200
15 C1b C 23.8000 -18.6200
16 C1b C 22.5400 -19.3200
17 O7a O 21.3500 -18.6200
18 C7a C 20.0900 -19.2500
19 C1c C 18.9000 -18.6200
20 C1c C 17.7100 -19.2500
21 C1a C 16.5200 -18.5500
22 C1a C 17.7100 -20.7200
23 O6a O 20.0900 -20.7200
24 N1a N 18.9000 -17.2200
BOND 24
1 2 3 1
2 3 4 2
3 4 5 1
4 5 6 2
5 2 6 1
6 3 7 1
7 7 8 2
8 8 9 1
9 9 10 1
10 4 10 1
11 10 11 2
12 8 12 1
13 2 13 1
14 13 14 1
15 14 15 1
16 15 16 1
17 16 17 1
18 17 18 1
19 18 19 1
20 19 20 1
21 20 21 1
22 20 22 1
23 18 23 2
24 19 24 1 #Down
|
|---|
|
» Japanese version » Back
|