 | | DRUG: Acyclovir | |
| Entry |
|
|---|
| Name |
Acyclovir (USP);
Aciclovir (JP18/INN);
Sitavig (TN);
Zovirax (TN) |
|---|
| Product |
|
|---|
| Generic |
ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (ACTAVIS PHARMA), ACYCLOVIR (ATLANTIC BIOLOGICALS CORP.), ACYCLOVIR (Actavis Pharma), ACYCLOVIR (Advagen Pharma Limited), ACYCLOVIR (Advanced Rx Pharmacy of Tennessee), ACYCLOVIR (Alembic Pharmaceuticals), ACYCLOVIR (Alembic Pharmaceuticals), ACYCLOVIR (American Health Packaging), ACYCLOVIR (American Health Packaging), ACYCLOVIR (American Health Packaging), ACYCLOVIR (Amneal Pharmaceuticals LLC), ACYCLOVIR (Amneal Pharmaceuticals LLC), ACYCLOVIR (Apotex Corp.), ACYCLOVIR (Apotex Corp.), ACYCLOVIR (Asclemed USA), ACYCLOVIR (Aurobindo Pharma Limited), ACYCLOVIR (AvKARE), ACYCLOVIR (AvPAK), ACYCLOVIR (Bionpharma), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Cadila Pharmaceuticals Limited), ACYCLOVIR (Cadila Pharmaceuticals Limited), ACYCLOVIR (Camber Pharmaceuticals), ACYCLOVIR (Camber Pharmaceuticals), ACYCLOVIR (Cardinal Health 107), ACYCLOVIR (Cardinal Health 107), ACYCLOVIR (Carlsbad Technology), ACYCLOVIR (Carlsbad Technology), ACYCLOVIR (Chartwell RX), ACYCLOVIR (Chartwell RX), ACYCLOVIR (Cipla USA), ACYCLOVIR (Coupler LLC), ACYCLOVIR (Coupler LLC), ACYCLOVIR (Coupler LLC), ACYCLOVIR (Coupler LLC), ACYCLOVIR (EMC PHARMA), ACYCLOVIR (Glenmark Pharmaceuticals), ACYCLOVIR (Golden State Medical Supply), ACYCLOVIR (Golden State Medical Supply), ACYCLOVIR (Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals), ACYCLOVIR (Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals), ACYCLOVIR (Macleods Pharmaceuticals Limited), ACYCLOVIR (Major Pharmaceuticals), ACYCLOVIR (Major Pharmaceuticals), ACYCLOVIR (Major Pharmaceuticals), ACYCLOVIR (Modavar Pharmaceuticals LLC), ACYCLOVIR (Modavar Pharmaceuticals LLC), ACYCLOVIR (Mylan Pharmaceuticals), ACYCLOVIR (Nivagen Pharmaceuticals), ACYCLOVIR (Nivagen Pharmaceuticals), ACYCLOVIR (Northstar Rx LLC), ACYCLOVIR (Northwind Pharmaceuticals), ACYCLOVIR (Northwind Pharmaceuticals), ACYCLOVIR (Northwind Pharmaceuticals), ACYCLOVIR (Novitium Pharma LLC), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (PD-Rx Pharmaceuticals), ACYCLOVIR (PD-Rx Pharmaceuticals), ACYCLOVIR (PD-Rx Pharmaceuticals), ACYCLOVIR (Padagis Israel Pharmaceuticals), ACYCLOVIR (PharmPak), ACYCLOVIR (PharmPak), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Quinn Pharmaceuticals), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (Redpharm Drug), ACYCLOVIR (Redpharm Drug), ACYCLOVIR (Redpharm drugs), ACYCLOVIR (SQUARE PHARMACEUTICALS LIMITED), ACYCLOVIR (ST. MARY'S MEDICAL PARK PHARMACY), ACYCLOVIR (Solco Healthcare US), ACYCLOVIR (St. Mary's Medical Park Pharmacy), ACYCLOVIR (Sun Pharmaceutical Industries), ACYCLOVIR (Sun Pharmaceutical Industries), ACYCLOVIR (Teva Pharmaceuticals USA), ACYCLOVIR (Torrent Pharmaceuticals Limited), ACYCLOVIR (Viona Pharmaceuticals), ACYCLOVIR (Viona Pharmaceuticals), ACYCLOVIR (Viona Pharmaceuticals), ACYCLOVIR (VistaPharm), ACYCLOVIR (Westminster Pharmaceuticals), ACYCLOVIR (Xiromed), ACYCLOVIR (YILING PHARMACEUTICAL), ACYCLOVIR (YILING PHARMACEUTICAL), ACYCLOVIR (YILING PHARMACEUTICAL), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Pharmaceuticals (USA)), ACYCLOVIR SUSPENSION (Novadoz Pharmaceuticals LLC) |
|---|
| Formula |
C8H11N5O3
|
|---|
| Exact mass |
225.0862
|
|---|
| Mol weight |
225.21
|
|---|
| Structure |
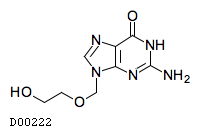
|
|---|
| Simcomp |
|
|---|
| Class |
Antiviral
DG02840 Anti-herpesvirus agent
Transporter substrate
DG02858 SLC22A1 substrate
DG02859 SLC22A6 substrate
DG02860 SLC22A8 substrate
|
|---|
| Remark |
| Product (DG00406): | D00222<JP/US> D02764<US> |
| Product (mixture): | D11627<US> |
|
|---|
| Efficacy |
Antiviral, DNA polymerase inhibitor |
|---|
| Disease |
Herpes simplex virus infection [DS: H00365] Herpes zoster infection [DS: H00366] Chickenpox [DS: H00366] |
|---|
| Target |
Herpesvirus DNA polymerase [KO: K18964] |
|---|
| Pathway |
|
|---|
| Metabolism |
Transporter: SLC22A1 [HSA: 6580], SLC22A6 [HSA: 9356], SLC22A8 [HSA: 9376]
|
|---|
| Interaction |
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
D DERMATOLOGICALS
D06 ANTIBIOTICS AND CHEMOTHERAPEUTICS FOR DERMATOLOGICAL USE
D06B CHEMOTHERAPEUTICS FOR TOPICAL USE
D06BB Antivirals
D06BB03 Aciclovir
D00222 Acyclovir (USP) <JP/US>
J ANTIINFECTIVES FOR SYSTEMIC USE
J05 ANTIVIRALS FOR SYSTEMIC USE
J05A DIRECT ACTING ANTIVIRALS
J05AB Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB01 Aciclovir
D00222 Acyclovir (USP) <JP/US>
S SENSORY ORGANS
S01 OPHTHALMOLOGICALS
S01A ANTIINFECTIVES
S01AD Antivirals
S01AD03 Aciclovir
D00222 Acyclovir (USP) <JP/US>
USP drug classification [BR:br08302]
Antivirals
Antiherpetic Agents
Acyclovir
D00222 Acyclovir (USP)
Dermatological Agents
Topical Anti-infectives
Antivirals, Dermatological
Acyclovir
D00222 Acyclovir (USP)
Therapeutic category of drugs in Japan [BR:br08301]
1 Agents affecting nervous system and sensory organs
13 Agents affecting sensory organs
131 Ophthalmic agents
1319 Others
D00222 Acyclovir (USP); Aciclovir (JP18/INN)
6 Agents against pathologic organisms and parasites
62 Chemotherapeutics
625 Antivirals
6250 Antivirals
D00222 Acyclovir (USP); Aciclovir (JP18/INN)
Classification of Japanese OTC drugs [BR:br08313]
Agents for integumentary system
91 Antivirals
D00222 Acyclovir (USP)
Risk category of Japanese OTC drugs [BR:br08312]
First-class OTC drugs
Inorganic and organic chemicals
Aciclovir
D00222 Acyclovir (USP)
Drug groups [BR:br08330]
Antiviral
DG02840 Anti-herpesvirus agent
DG00406 Aciclovir
D00222 Acyclovir
Transporter substrate
DG02858 SLC22A1 substrate
DG00406 Aciclovir
D00222 Acyclovir
DG02859 SLC22A6 substrate
DG00406 Aciclovir
D00222 Acyclovir
DG02860 SLC22A8 substrate
DG00406 Aciclovir
D00222 Acyclovir
Drug classes [BR:br08332]
Antiviral
DG02840 Anti-herpesvirus agent
D00222 Acyclovir
Antimicrobials [BR:br08307]
Antivirals
Genome replication inhibitor
Herpesvirus DNA polymerase inhibitor
D00222 Acyclovir (USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00222 Aciclovir
D00222 Aciclovir tablets
D00222 Aciclovir granules
D00222 Aciclovir syrup
D00222 Aciclovir for syrup
D00222 Aciclovir injection
D00222 Aciclovir for injection
D00222 Aciclovir ophthalmic oinment
D00222 Aciclovir ointment
Drug metabolizing enzymes and transporters [br08309.html]
Drug transporters
D00222
Prodrugs [br08324.html]
D00222
Rx-to-OTC switch list in Japan [br08314.html]
D00222
Drug groups [BR:br08330]
Antiviral
DG02840 Anti-herpesvirus agent
DG00406 Aciclovir
Transporter substrate
DG02858 SLC22A1 substrate
DG00406 Aciclovir
DG02859 SLC22A6 substrate
DG00406 Aciclovir
DG02860 SLC22A8 substrate
DG00406 Aciclovir
Antimicrobials abbreviations [BR:br08327]
Antivirals
Genome replication inhibitor
Herpesvirus DNA polymerase inhibitor
DG00406 Aciclovir
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 16
1 N4y N 23.9021 -15.7728
2 C8y C 25.2251 -15.3140
3 C8y C 25.1976 -13.9138
4 N5x N 23.8575 -13.5074
5 C8x C 23.0568 -14.6564
6 N5x N 26.4513 -15.9900
7 C8y C 27.6500 -15.2662
8 N4x N 27.6224 -13.8662
9 C8y C 26.3961 -13.1901
10 O5x O 26.3689 -11.8062
11 N1a N 28.8860 -15.9474
12 C1b C 23.9021 -17.1728
13 O2a O 22.6761 -17.8812
14 C1b C 21.4530 -17.1758
15 C1b C 20.2280 -17.8843
16 O1a O 19.0018 -17.1780
BOND 17
1 1 2 1
2 2 3 2
3 3 4 1
4 4 5 2
5 1 5 1
6 2 6 1
7 6 7 2
8 7 8 1
9 8 9 1
10 3 9 1
11 9 10 2
12 7 11 1
13 1 12 1
14 12 13 1
15 13 14 1
16 14 15 1
17 15 16 1
|
|---|
|
» Japanese version » Back
|