 | | DRUG: Enalapril maleate | |
| Entry |
|
|---|
| Name |
Enalapril maleate (JP18/USP);
Renivace (TN);
Vasotec (TN) |
|---|
| Product |
|
|---|
| Generic |
ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (A-S Medication Solutions), ENALAPRIL MALEATE (Amici Pharma), ENALAPRIL MALEATE (Amneal Pharmaceuticals NY LLC), ENALAPRIL MALEATE (Amneal Pharmaceuticals NY LLC), ENALAPRIL MALEATE (Aurobindo Pharma Limited), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Bryant Ranch Prepack), ENALAPRIL MALEATE (Chartwell RX), ENALAPRIL MALEATE (Coupler LLC), ENALAPRIL MALEATE (DirectRx), ENALAPRIL MALEATE (Golden State Medical Supply), ENALAPRIL MALEATE (Golden State Medical Supply), ENALAPRIL MALEATE (Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals), ENALAPRIL MALEATE (Legacy Pharmaceutical Packaging), ENALAPRIL MALEATE (Legacy Pharmaceutical Packaging), ENALAPRIL MALEATE (Major Pharmaceuticals), ENALAPRIL MALEATE (Northstar RxLLC), ENALAPRIL MALEATE (Northwind Pharmaceuticals), ENALAPRIL MALEATE (Northwind Pharmaceuticals), ENALAPRIL MALEATE (Northwind Pharmaceuticals), ENALAPRIL MALEATE (Northwind Pharmaceuticals), ENALAPRIL MALEATE (Northwind Pharmaceuticals), ENALAPRIL MALEATE (NuCare Pharmaceuticals), ENALAPRIL MALEATE (NuCare Pharmaceuticals), ENALAPRIL MALEATE (NuCare Pharmaceuticals), ENALAPRIL MALEATE (NuCare Pharmaceuticals), ENALAPRIL MALEATE (NuCare Pharmaceuticals), ENALAPRIL MALEATE (OCEANSIDE PHARMACEUTICALS), ENALAPRIL MALEATE (PD-Rx Pharmaceuticals), ENALAPRIL MALEATE (PD-Rx Pharmaceuticals), ENALAPRIL MALEATE (Preferred Pharmaceuicals), ENALAPRIL MALEATE (Preferred Pharmaceuticals), ENALAPRIL MALEATE (Preferred Pharmaceuticals), ENALAPRIL MALEATE (Preferred Pharmaceuticals), ENALAPRIL MALEATE (Proficient Rx LP), ENALAPRIL MALEATE (Proficient Rx LP), ENALAPRIL MALEATE (Proficient Rx LP), ENALAPRIL MALEATE (Proficient Rx LP), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (REMEDYREPACK), ENALAPRIL MALEATE (Rising Pharma Holdings), ENALAPRIL MALEATE (ST. MARY'S MEDICAL PARK PHARMACY), ENALAPRIL MALEATE (ScieGen Pharmaceuticals), ENALAPRIL MALEATE (Solco Healthcare US), ENALAPRIL MALEATE (St Mary's Medical Park Pharmacy), ENALAPRIL MALEATE (St Marys Medical Park Pharmacy), ENALAPRIL MALEATE (St. Mary's Medical Park Pharmacy), ENALAPRIL MALEATE (Taro Pharmaceuticals U.S.A.), ENALAPRIL MALEATE ORAL SOLUTION (Ascend Laboratories), ENALAPRIL MALEATE ORAL SOLUTION (Bionpharma), ENALAPRIL MALEATE ORAL SOLUTION (Camber Pharmaceuticals), ENALAPRIL MALEATE ORAL SOLUTION (NorthStar RxLLC) |
|---|
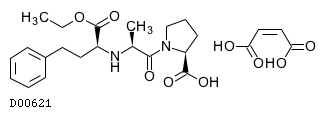
| Formula |
C20H28N2O5. C4H4O4
|
|---|
| Exact mass |
492.2108
|
|---|
| Mol weight |
492.52
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
Cardiovascular agent
DG01925 Renin-angiotensin system inhibitor
DG01501 Angiotensin-converting enzyme inhibitor
DG03231 Antihypertensive
DG01501 Angiotensin-converting enzyme inhibitor
|
|---|
| Remark |
| Product (DG00335): | D00621<JP/US> |
| Product (mixture): | D10277<US> |
|
|---|
| Efficacy |
Antihypertensive, Vasodilator, Angiotensin-converting enzyme inhibitor |
|---|
| Disease |
|
|---|
| Comment |
Active form of prodrug: Enalaprilat [CPD: C11720]
|
|---|
| Target |
|
|---|
| Pathway |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07217 | Renin-angiotensin system inhibitors |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
C CARDIOVASCULAR SYSTEM
C09 AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
C09A ACE INHIBITORS, PLAIN
C09AA ACE inhibitors, plain
C09AA02 Enalapril
D00621 Enalapril maleate (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Cardiovascular Agents
Angiotensin-converting Enzyme (ACE) Inhibitors
Enalapril
D00621 Enalapril maleate (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
21 Cardiovascular agents
214 Antihypertensives
2144 Angiotensin converting enzyme inhibitors
D00621 Enalapril maleate (JP18/USP)
217 Vasodilators
2179 Others
D00621 Enalapril maleate (JP18/USP)
Drug groups [BR:br08330]
Cardiovascular agent
DG01925 Renin-angiotensin system inhibitor
DG01501 Angiotensin-converting enzyme inhibitor
DG00335 Enalapril
D00621 Enalapril maleate
DG03231 Antihypertensive
DG01501 Angiotensin-converting enzyme inhibitor
DG00335 Enalapril
D00621 Enalapril maleate
Drug classes [BR:br08332]
Cardiovascular agent
DG01501 Angiotensin-converting enzyme inhibitor
D00621 Enalapril maleate
DG03231 Antihypertensive
D00621 Enalapril maleate
Target-based classification of drugs [BR:br08310]
Peptidases and inhibitors
Metallo peptidases
Family M2
ACE (CD143)
D00621 Enalapril maleate (JP18/USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00621 Enalapril maleate
D00621 Enalapril maleate tablets
New drug approvals in Europe [br08329.html]
European public assessment reports (EPAR) authorised medicine
D00621
Drug groups [BR:br08330]
Cardiovascular agent
DG01925 Renin-angiotensin system inhibitor
DG01501 Angiotensin-converting enzyme inhibitor
DG00335 Enalapril
DG03231 Antihypertensive
DG01501 Angiotensin-converting enzyme inhibitor
DG00335 Enalapril
Prodrugs [br08324.html]
DG00335
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 35
1 N1y N 30.6132 -16.0047
2 C1x C 30.6011 -14.5972
3 C5a C 29.4025 -16.7276
4 C1y C 31.9477 -16.4196
5 C1x C 31.9318 -14.1523
6 C1c C 28.1801 -16.0341
7 O5a O 29.4201 -18.1324
8 C1x C 32.7630 -15.2776
9 N1b N 26.9870 -16.7571
10 C1a C 28.1684 -14.6294
11 C1c C 25.7645 -16.0576
12 C1b C 24.5538 -16.7806
13 C7a C 25.7527 -14.6588
14 C1b C 23.3372 -16.0871
15 O7a O 24.5303 -13.9770
16 O6a O 26.9458 -13.9535
17 C8y C 22.1264 -16.8099
18 C8x C 20.8948 -16.1226
19 C8x C 19.6927 -16.8403
20 C8x C 19.6432 -18.2401
21 C8x C 20.9448 -18.9274
22 C8x C 22.1469 -18.2098
23 C1b C 23.2951 -14.7129
24 C1a C 22.0792 -14.0328
25 C6a C 31.9477 -17.8196
26 O6a O 30.7012 -18.5388
27 O6a O 33.1518 -18.5153
28 C2b C 38.6529 -14.8470
29 C2b C 37.0191 -14.8528
30 C6a C 39.3289 -16.0166
31 C6a C 36.3431 -16.0284
32 O6a O 38.6589 -17.1920
33 O6a O 40.6924 -16.0049
34 O6a O 34.9855 -16.0284
35 O6a O 37.0308 -17.2038
BOND 35
1 1 2 1
2 1 3 1
3 1 4 1
4 2 5 1
5 3 6 1
6 3 7 2
7 4 8 1
8 6 9 1
9 6 10 1 #Up
10 9 11 1
11 11 12 1
12 11 13 1 #Up
13 12 14 1
14 13 15 1
15 13 16 2
16 14 17 1
17 5 8 1
18 17 18 2
19 18 19 1
20 19 20 2
21 20 21 1
22 21 22 2
23 17 22 1
24 15 23 1
25 23 24 1
26 4 25 1 #Up
27 25 26 2
28 25 27 1
29 28 29 2
30 28 30 1
31 29 31 1
32 30 32 1
33 30 33 2
34 31 34 1
35 31 35 2
|
|---|
|
» Japanese version » Back
|