| Entry |
|
|---|
| Name |
Indole diterpene alkaloid biosynthesis |
|---|
| Description |
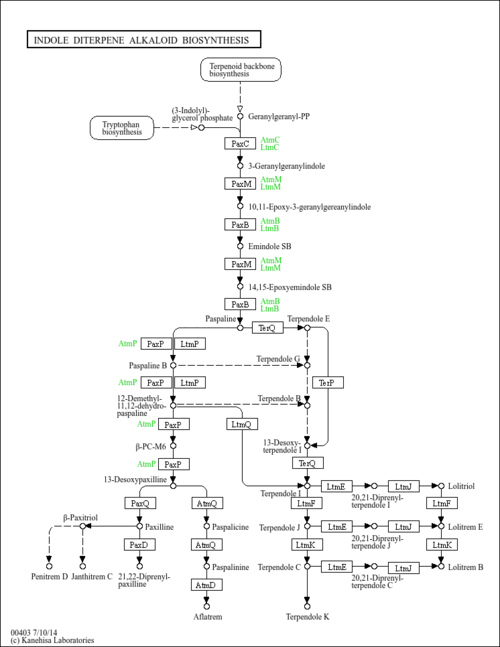
Indole diterpene alkaloids are a group of meroterpenoids consisting of a partial diterpene structure with four isoprene units and an indole moiety. The common cyclic diterpene skeleton is derived from geranylgeranyl diphosphate (GGPP) and an indole moiety is derived from tryptophan or a tryptophan precursor. They are combined in the initial step of biosynthetic reactions that generate paspaline, the first stable and less modified form required for other forms. Most indole diterpene alkaloids have potent mammalian tremorgenic activities, such as ryegrass staggers in livestock, and some have anti-insect properties. This map shows examples of indole diterpene alkaloids isolated from filamentous fungi.
|
|---|
| Class |
Metabolism; Biosynthesis of other secondary metabolites
|
|---|
| Pathway map |
| rn00403 | Indole diterpene alkaloid biosynthesis |
|
|---|
| Module |
| M00661 | Paspaline biosynthesis, geranylgeranyl-PP + indoleglycerol phosphate => paspaline [PATH:rn00403] |
|
|---|
| Reaction |
| R10350 | 3-geranylgeranylindole,NADPH:oxygen oxidoreductase (10,11-epoxidizing) |
| R10351 | emindole-SB,NADPH:oxygen oxidoreductase (14,15-epoxidizing) |
| R10356 | paspaline-B,NADPH:oxygen oxidoreductase |
| R10359 | 13-desoxypaxilline,NADPH:oxygen oxidoreductase |
| R10365 | dimethylallyl-diphosphate:paspalinine dimethylallyltransferase |
| R10366 | 13-desoxyterpendole I,NADPH:oxygen oxidoreductase |
| R10370 | dimethylallyl-diphosphate:lolitriol dimethylallyl-O-transferase |
| R10371 | paspaline,NADPH:oxygen oxidoreductase |
| R10373 | paspaline,NADPH:oxygen oxidoreductase |
| R10380 | dimethylallyl-diphosphate:terpendole-I dimethylallyl-O-transferase |
|
|---|
| Compound |
| C00353 | Geranylgeranyl diphosphate |
| C03506 | Indoleglycerol phosphate |
| C20526 | 10,11-Epoxy-3-geranylgeranylindole |
| C20532 | 20,21-Diprenylterpendole I |
| C20534 | 12-Demethyl-11,12-dehydropaspaline |
| C20561 | 21,22-Diprenylpaxilline |
| C20594 | 20,21-Diprenylterpendole J |
| C20595 | 20,21-Diprenylterpendole C |
|
|---|
| Reference |
|
|---|
| Authors |
Saikia S, Nicholson MJ, Young C, Parker EJ, Scott B |
|---|
| Title |
The genetic basis for indole-diterpene chemical diversity in filamentous fungi. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Saikia S, Parker EJ, Koulman A, Scott B |
|---|
| Title |
Four gene products are required for the fungal synthesis of the indole-diterpene, paspaline. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Saikia S, Parker EJ, Koulman A, Scott B |
|---|
| Title |
Defining paxilline biosynthesis in Penicillium paxilli: functional characterization of two cytochrome P450 monooxygenases. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Nicholson MJ, Koulman A, Monahan BJ, Pritchard BL, Payne GA, Scott B |
|---|
| Title |
Identification of two aflatrem biosynthesis gene loci in Aspergillus flavus and metabolic engineering of Penicillium paxilli to elucidate their function. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Saikia S, Takemoto D, Tapper BA, Lane GA, Fraser K, Scott B |
|---|
| Title |
Functional analysis of an indole-diterpene gene cluster for lolitrem B biosynthesis in the grass endosymbiont Epichloe festucae. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Motoyama T, Hayashi T, Hirota H, Ueki M, Osada H |
|---|
| Title |
Terpendole E, a kinesin Eg5 inhibitor, is a key biosynthetic intermediate of indole-diterpenes in the producing fungus Chaunopycnis alba. |
|---|
| Journal |
|
|---|
Related
pathway |
| rn00400 | Phenylalanine, tyrosine and tryptophan biosynthesis |
| rn00900 | Terpenoid backbone biosynthesis |
|
|---|
| KO pathway |
|
|---|