 | | DRUG: アシクロビル | |
| エントリ |
|
|---|
| 一般名 |
アシクロビル (JP18);
Acyclovir (USP);
Aciclovir (JP18/INN) |
|---|
| 商品名 |
アシクロビル (ロートニッテン), アシクロビル (日医工), アシクロビル (日医工岐阜工場), アシクロビル (東亜薬品), アシクロビル (東和薬品), アシクロビル (東和薬品), アシクロビル (沢井製薬), アシクロビル (沢井製薬), アシクロビル (長生堂製薬), ゾビラックス (グラクソ・スミスクライン), ゾビラックス (グラクソ・スミスクライン), ゾビラックス (グラクソ・スミスクライン), ゾビラックス (グラクソ・スミスクライン), ゾビラックス (グラクソ・スミスクライン), ゾビラックス (日東メディック), ゾビラックス (日東メディック) |
|---|
| 後発品 |
アシクロビル (ネオクリティケア製薬), アシクロビル (日医工), アシクロビル (日医工), アシクロビル (日本化薬), アシクロビル (東光薬品工業), アシクロビル (東和薬品), アシクロビル (東和薬品), アシクロビル (沢井製薬), アシクロビル (沢井製薬), アシクロビル (長生堂製薬), アシクロビル (高田製薬), アシクロビル (高田製薬) |
|---|
| 米国の商品 |
|
|---|
| 後発品 |
ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (A-S Medication Solutions), ACYCLOVIR (ACTAVIS PHARMA), ACYCLOVIR (ATLANTIC BIOLOGICALS CORP.), ACYCLOVIR (Actavis Pharma), ACYCLOVIR (Advagen Pharma Limited), ACYCLOVIR (Advanced Rx Pharmacy of Tennessee), ACYCLOVIR (Alembic Pharmaceuticals), ACYCLOVIR (Alembic Pharmaceuticals), ACYCLOVIR (American Health Packaging), ACYCLOVIR (American Health Packaging), ACYCLOVIR (American Health Packaging), ACYCLOVIR (Amneal Pharmaceuticals LLC), ACYCLOVIR (Amneal Pharmaceuticals LLC), ACYCLOVIR (Apotex Corp.), ACYCLOVIR (Apotex Corp.), ACYCLOVIR (Asclemed USA), ACYCLOVIR (Aurobindo Pharma Limited), ACYCLOVIR (Aurobindo Pharma Limited), ACYCLOVIR (AvKARE), ACYCLOVIR (AvPAK), ACYCLOVIR (Bionpharma), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Bryant Ranch Prepack), ACYCLOVIR (Cadila Pharmaceuticals Limited), ACYCLOVIR (Cadila Pharmaceuticals Limited), ACYCLOVIR (Camber Pharmaceuticals), ACYCLOVIR (Camber Pharmaceuticals), ACYCLOVIR (Cardinal Health 107), ACYCLOVIR (Cardinal Health 107), ACYCLOVIR (Carlsbad Technology), ACYCLOVIR (Carlsbad Technology), ACYCLOVIR (Chartwell RX), ACYCLOVIR (Chartwell RX), ACYCLOVIR (Cipla USA), ACYCLOVIR (Coupler LLC), ACYCLOVIR (Coupler LLC), ACYCLOVIR (Coupler LLC), ACYCLOVIR (Coupler LLC), ACYCLOVIR (Direct_Rx), ACYCLOVIR (EMC PHARMA), ACYCLOVIR (Glenmark Pharmaceuticals), ACYCLOVIR (Golden State Medical Supply), ACYCLOVIR (Golden State Medical Supply), ACYCLOVIR (Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals), ACYCLOVIR (Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals), ACYCLOVIR (Macleods Pharmaceuticals Limited), ACYCLOVIR (Major Pharmaceuticals), ACYCLOVIR (Major Pharmaceuticals), ACYCLOVIR (Major Pharmaceuticals), ACYCLOVIR (Modavar Pharmaceuticals LLC), ACYCLOVIR (Modavar Pharmaceuticals LLC), ACYCLOVIR (Mylan Pharmaceuticals), ACYCLOVIR (Nivagen Pharmaceuticals), ACYCLOVIR (Nivagen Pharmaceuticals), ACYCLOVIR (Northstar Rx LLC), ACYCLOVIR (Northwind Pharmaceuticals), ACYCLOVIR (Northwind Pharmaceuticals), ACYCLOVIR (Northwind Pharmaceuticals), ACYCLOVIR (Novitium Pharma LLC), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (NuCare Pharmaceuticals), ACYCLOVIR (PD-Rx Pharmaceuticals), ACYCLOVIR (PD-Rx Pharmaceuticals), ACYCLOVIR (PD-Rx Pharmaceuticals), ACYCLOVIR (Padagis Israel Pharmaceuticals), ACYCLOVIR (PharmPak), ACYCLOVIR (PharmPak), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Preferred Pharmaceuticals), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Proficient Rx LP), ACYCLOVIR (Quinn Pharmaceuticals), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (REMEDYREPACK), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (RedPharm Drug), ACYCLOVIR (Redpharm Drug), ACYCLOVIR (Redpharm Drug), ACYCLOVIR (Redpharm drugs), ACYCLOVIR (SQUARE PHARMACEUTICALS LIMITED), ACYCLOVIR (ST. MARY'S MEDICAL PARK PHARMACY), ACYCLOVIR (Solco Healthcare US), ACYCLOVIR (St. Mary's Medical Park Pharmacy), ACYCLOVIR (Sun Pharmaceutical Industries), ACYCLOVIR (Sun Pharmaceutical Industries), ACYCLOVIR (Teva Pharmaceuticals USA), ACYCLOVIR (Torrent Pharmaceuticals Limited), ACYCLOVIR (Viona Pharmaceuticals), ACYCLOVIR (Viona Pharmaceuticals), ACYCLOVIR (Viona Pharmaceuticals), ACYCLOVIR (Westminster Pharmaceuticals), ACYCLOVIR (Xiromed), ACYCLOVIR (YILING PHARMACEUTICAL), ACYCLOVIR (YILING PHARMACEUTICAL), ACYCLOVIR (YILING PHARMACEUTICAL), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Lifesciences Limited), ACYCLOVIR (Zydus Pharmaceuticals (USA)), ACYCLOVIR SUSPENSION (Novadoz Pharmaceuticals LLC) |
|---|
| 組成式 |
C8H11N5O3
|
|---|
| 質量 |
225.0862
|
|---|
| 分子量 |
225.21
|
|---|
| 構造式 |
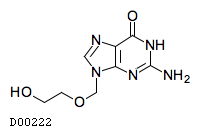
|
|---|
| Simcomp |
|
|---|
| クラス |
|
|---|
| コード |
| 商品 (DG00406): | D00222<JP/US> D02764<US> |
|
|---|
| 効能 |
抗ウイルス薬, DNAポリメラーゼ阻害薬 |
|---|
| 疾患 |
|
|---|
| ターゲット |
Herpesvirus DNA polymerase [KO: K18964] |
|---|
| パスウェイ |
|
|---|
| 代謝 |
Transporter: SLC22A1 [HSA: 6580], SLC22A6 [HSA: 9356], SLC22A8 [HSA: 9376]
|
|---|
| 相互作用 |
|
|---|
| 構造マップ |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
1 神経系及び感覚器官用医薬品
13 感覚器官用薬
131 眼科用剤
1319 その他の眼科用剤
D00222 アシクロビル (JP18)
6 病原生物に対する医薬品
62 化学療法剤
625 抗ウイルス剤
6250 抗ウイルス剤
D00222 アシクロビル (JP18)
医療用医薬品のATC分類 [BR:jp08303]
D 皮膚科用薬
D06 皮膚科用抗生物質と化学療法薬
D06B 局所用化学療法薬
D06BB 抗ウイルス薬
D06BB03 アシクロビル
D00222 アシクロビル (JP18) <JP/US>
J 全身用抗感染薬
J05 全身用抗ウイルス薬
J05A 直接作用型抗ウイルス薬
J05AB ヌクレオシドとヌクレオチド、逆転写酵素阻害薬を除く
J05AB01 アシクロビル
D00222 アシクロビル (JP18) <JP/US>
S 感覚器
S01 眼科用薬
S01A 抗感染薬
S01AD 抗ウイルス薬
S01AD03 アシクロビル
D00222 アシクロビル (JP18) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
抗ウイルス薬
抗ヘルペス薬
アシクロビル
D00222 アシクロビル (JP18)
皮膚科用薬
外用抗感染症薬
抗ウイルス薬, 皮膚科用
アシクロビル
D00222 アシクロビル (JP18)
一般用医薬品の分類 [BR:jp08313]
外皮用薬
91 抗ウイルス薬
D00222 アシクロビル (JP18)
一般用医薬品のリスク区分 [BR:jp08312]
第一類医薬品
無機薬品及び有機薬品
アシクロビル
D00222 アシクロビル (JP18)
医薬品グループ [BR:jp08330]
抗ウイルス薬
DG02840 抗ヘルペスウイルス薬
DG00406 アシクロビル
D00222 アシクロビル
トランスポーター基質薬
DG02858 SLC22A1基質薬
DG00406 アシクロビル
D00222 アシクロビル
DG02859 SLC22A6基質薬
DG00406 アシクロビル
D00222 アシクロビル
DG02860 SLC22A8基質薬
DG00406 アシクロビル
D00222 アシクロビル
医薬品クラス [BR:jp08332]
抗ウイルス薬
DG02840 抗ヘルペスウイルス薬 [商品一覧]
D00222 アシクロビル
抗微生物薬 [BR:jp08307]
抗ウイルス薬
ゲノム複製阻害薬
ヘルペスウイルスDNAポリメラーゼ阻害薬
D00222 アシクロビル (JP18) <JP/US>
日本薬局方収載医薬品 [BR:jp08311]
化学薬品等
D00222 アシクロビル
D00222 アシクロビル錠
D00222 アシクロビル顆粒
D00222 アシクロビルシロップ
D00222 シロップ用アシクロビル
D00222 アシクロビル注射液
D00222 注射用アシクロビル
D00222 アシクロビル眼軟膏
D00222 アシクロビル軟膏
薬物代謝酵素とトランスポーター [jp08309.html]
薬物トランスポーター
D00222
日本のスイッチOTC薬 [jp08314.html]
医療用から一般用へのスイッチOTC薬
D00222
医薬品グループ [BR:jp08330]
抗ウイルス薬
DG02840 抗ヘルペスウイルス薬
DG00406 アシクロビル
トランスポーター基質薬
DG02858 SLC22A1基質薬
DG00406 アシクロビル
DG02859 SLC22A6基質薬
DG00406 アシクロビル
DG02860 SLC22A8基質薬
DG00406 アシクロビル
抗微生物薬の略語 [BR:jp08327]
抗ウイルス薬
ゲノム複製阻害薬
ヘルペスウイルスDNAポリメラーゼ阻害薬
DG00406 アシクロビル
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|