 | | DRUG: Fenofibrate | |
| Entry |
|
|---|
| Name |
Fenofibrate (JP18/USP/INN);
Antara (TN);
Lipantil (TN);
Lipofen (TN);
Tricor (TN);
Triglide (TN) |
|---|
| Product |
|
|---|
| Generic |
FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (A-S Medication Solutions), FENOFIBRATE (ANI Pharmaceuticals), FENOFIBRATE (ANI Pharmaceuticals), FENOFIBRATE (Ajanta Pharma USA), FENOFIBRATE (Ajanta Pharma USA), FENOFIBRATE (Alembic Pharmaceuticals Limited), FENOFIBRATE (Alembic Pharmaceuticals Limited), FENOFIBRATE (Alembic Pharmaceuticals Limited), FENOFIBRATE (Alembic Pharmaceuticals Limited), FENOFIBRATE (Alembic Pharmaceuticals), FENOFIBRATE (Alembic Pharmaceuticals), FENOFIBRATE (Alembic Pharmaceuticals), FENOFIBRATE (Alembic Pharmaceuticals), FENOFIBRATE (Alembic Pharmaceuticals), FENOFIBRATE (American Health Packaging), FENOFIBRATE (American Health Packaging), FENOFIBRATE (American Health Packaging), FENOFIBRATE (Amneal Pharmaceuticals NY LLC), FENOFIBRATE (Amneal Pharmaceuticals NY LLC), FENOFIBRATE (Amneal Pharmaceuticals NY LLC), FENOFIBRATE (Amneal Pharmaceuticals of New York LLC), FENOFIBRATE (Aphena Pharma Solutions - Tennessee), FENOFIBRATE (Apotex Corp.), FENOFIBRATE (Aurobindo Pharma Limited), FENOFIBRATE (Aurobindo Pharma Limited), FENOFIBRATE (Aurobindo Pharma Limited), FENOFIBRATE (AustarPharma LLC), FENOFIBRATE (Austarpharma), FENOFIBRATE (AvKARE), FENOFIBRATE (AvKARE), FENOFIBRATE (AvPAK), FENOFIBRATE (AvPAK), FENOFIBRATE (Bostal LLC), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Bryant Ranch Prepack), FENOFIBRATE (Camber Pharmaceuticals), FENOFIBRATE (Chartwell RX), FENOFIBRATE (Chartwell RX), FENOFIBRATE (Cipla USA Inc.,), FENOFIBRATE (Cipla USA), FENOFIBRATE (Coupler LLC), FENOFIBRATE (Coupler LLC), FENOFIBRATE (Creekwood Pharmaceuticals LLC), FENOFIBRATE (Dr. Reddy's Laboratories Limited), FENOFIBRATE (Dr. Reddy's Laboratories), FENOFIBRATE (Glenmark Pharmaceuticals), FENOFIBRATE (Golden State Medical Supply), FENOFIBRATE (Golden State Medical Supply), FENOFIBRATE (Golden State Medical Supply), FENOFIBRATE (Laurus Labs Limited), FENOFIBRATE (Laurus Labs Limited), FENOFIBRATE (Leading Pharma), FENOFIBRATE (Leading Pharma), FENOFIBRATE (Lifestar Pharma LLC), FENOFIBRATE (Lupin Pharmaceuticals), FENOFIBRATE (Lupin Pharmaceuticals), FENOFIBRATE (Lupin Pharmaceuticals), FENOFIBRATE (Macleods Pharmaceuticals Limited), FENOFIBRATE (Major Pharmaceuticals), FENOFIBRATE (Major Pharmaceuticals), FENOFIBRATE (Major Pharmaceuticals), FENOFIBRATE (Mylan Institutional), FENOFIBRATE (Mylan Pharmaceuticals), FENOFIBRATE (Mylan Pharmaceuticals), FENOFIBRATE (NCS HealthCare of KY), FENOFIBRATE (NCS HealthCare of KY), FENOFIBRATE (NorthStar RxLLC), FENOFIBRATE (Northwind Pharmaceuticals), FENOFIBRATE (Northwind Pharmaceuticals), FENOFIBRATE (Northwind Pharmaceuticals), FENOFIBRATE (Northwind Pharmaceuticals), FENOFIBRATE (Northwind Pharmaceuticals), FENOFIBRATE (Northwind Pharmaceuticals), FENOFIBRATE (Northwind Pharmaceuticals), FENOFIBRATE (NuCare Pharmaceuticals), FENOFIBRATE (NuCare Pharmaceuticals), FENOFIBRATE (NuCare Pharmaceuticals), FENOFIBRATE (NuCare Pharmaceuticals), FENOFIBRATE (NuCare Pharmaceuticals), FENOFIBRATE (PD-Rx Pharmaceuticals), FENOFIBRATE (PD-Rx Pharmaceuticals), FENOFIBRATE (Preferred Pharmaceuticals), FENOFIBRATE (Preferred Pharmaceuticals), FENOFIBRATE (Preferred Pharmaceuticals), FENOFIBRATE (Preferred Pharmaceuticals), FENOFIBRATE (Preferred Pharmaceuticals), FENOFIBRATE (Proficient Rx LP), FENOFIBRATE (Proficient Rx LP), FENOFIBRATE (Proficient Rx LP), FENOFIBRATE (Proficient Rx LP), FENOFIBRATE (Proficient Rx LP), FENOFIBRATE (QUALLENT PHARMACEUTICALS HEALTH LLC), FENOFIBRATE (Quality Care Products), FENOFIBRATE (REMEDYREPACK), FENOFIBRATE (REMEDYREPACK), FENOFIBRATE (REMEDYREPACK), FENOFIBRATE (Rhodes Pharmaceuticals L.P.), FENOFIBRATE (Rhodes Pharmaceuticals L.P.), FENOFIBRATE (Solco Healthcare US), FENOFIBRATE (St. Mary's Medical Park Pharmacy), FENOFIBRATE (Sun Pharmaceutical Industries), FENOFIBRATE (Sun Pharmaceutical Industries), FENOFIBRATE (Torrent Pharmaceuticals Limited), FENOFIBRATE (Trifluent Pharma LLC), FENOFIBRATE (Westminster Pharmaceuticals) |
|---|
| Formula |
C20H21ClO4
|
|---|
| Exact mass |
360.1128
|
|---|
| Mol weight |
360.83
|
|---|
| Structure |
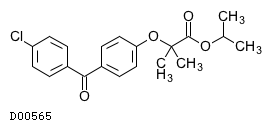
|
|---|
| Simcomp |
|
|---|
| Class |
Hypolipidemic agent
DG01946 Hypolipidemic agent
DG01733 PPAR alpha agonist
DG01547 Fibrate
Metabolizing enzyme substrate
DG02924 UGT substrate
|
|---|
| Remark |
| Therapeutic category: | 2183 |
| Product (DG01548): | D00565<JP/US> D08890<US> |
|
|---|
| Efficacy |
Antihyperlipidemic, Triglyceride synthesis inhibitor, Peroxisome proliferator-activated receptor (PPAR) alpha agonist |
|---|
| Disease |
Primary hypercholesterolemia or mixed dyslipidemia [DS: H01635] Hypertriglyceridemia [DS: H01637] |
|---|
| Comment |
Clofibrate derivative
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04920 | Adipocytokine signaling pathway |
|
|---|
| Metabolism |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07222 | Peroxisome proliferator-activated receptor (PPAR) agonists |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
C CARDIOVASCULAR SYSTEM
C10 LIPID MODIFYING AGENTS
C10A LIPID MODIFYING AGENTS, PLAIN
C10AB Fibrates
C10AB05 Fenofibrate
D00565 Fenofibrate (JP18/USP/INN) <JP/US>
USP drug classification [BR:br08302]
Cardiovascular Agents
Dyslipidemics, Fibric Acid Derivatives
Fenofibrate
D00565 Fenofibrate (JP18/USP/INN)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
21 Cardiovascular agents
218 Hyperlipidemia agents
2183 Clofibrates
D00565 Fenofibrate (JP18/USP/INN)
Drug groups [BR:br08330]
Hypolipidemic agent
DG01946 Hypolipidemic agent
DG01733 PPAR alpha agonist
DG01547 Fibrate
DG01548 Fenofibrate
D00565 Fenofibrate
Metabolizing enzyme substrate
DG02924 UGT substrate
DG01548 Fenofibrate
D00565 Fenofibrate
Drug classes [BR:br08332]
Cardiovascular agent
DG01946 Hypolipidemic agent
D00565 Fenofibrate
Target-based classification of drugs [BR:br08310]
Nuclear receptors
Thyroid hormone like receptors
Peroxisome proliferator-activated receptor (PPAR)
NR1C1 (PPARA)
D00565 Fenofibrate (JP18/USP/INN) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00565 Fenofibrate
D00565 Fenofibrate tablets
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00565
Drug groups [BR:br08330]
Hypolipidemic agent
DG01946 Hypolipidemic agent
DG01733 PPAR alpha agonist
DG01547 Fibrate
DG01548 Fenofibrate
Metabolizing enzyme substrate
DG02924 UGT substrate
DG01548 Fenofibrate
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 25
1 C8y C 29.4485 -16.2910
2 C5a C 28.2596 -16.9904
3 C8x C 30.7073 -16.9904
4 C8x C 29.4485 -14.8924
5 C8y C 27.0708 -16.2910
6 O5a O 28.2596 -18.3890
7 C8x C 31.8961 -16.2910
8 C8x C 30.7073 -14.1930
9 C8x C 25.8120 -16.9904
10 C8x C 27.0708 -14.8924
11 C8y C 31.8961 -14.8924
12 C8x C 24.6231 -16.2910
13 C8x C 25.8120 -14.1930
14 O2a O 33.0850 -14.1930
15 C8y C 24.6231 -14.8924
16 C1d C 34.3438 -14.8924
17 X Cl 23.4343 -14.1930
18 C7a C 35.5326 -14.1930
19 O7a O 36.7215 -14.8924
20 O6a O 35.5326 -12.7943
21 C1c C 37.9804 -14.1930
22 C1a C 39.1692 -14.8924
23 C1a C 37.9804 -12.7943
24 C1a C 33.6445 -16.1036
25 C1a C 35.0431 -16.1036
BOND 26
1 1 2 1
2 1 3 2
3 1 4 1
4 2 5 1
5 2 6 2
6 3 7 1
7 4 8 2
8 5 9 2
9 5 10 1
10 7 11 2
11 9 12 1
12 10 13 2
13 11 14 1
14 12 15 2
15 14 16 1
16 15 17 1
17 16 18 1
18 18 19 1
19 18 20 2
20 19 21 1
21 21 22 1
22 21 23 1
23 8 11 1
24 13 15 1
25 16 24 1
26 16 25 1
|
|---|
|
» Japanese version » Back
|