| Entry |
|
|---|
| Name |
Terbinafine hydrochloride (JP18/USP);
Lamisil (TN) |
|---|
| Product |
|
|---|
| Generic |
TERBINAFINE (A-S Medication Solutions), TERBINAFINE (A-S Medication Solutions), TERBINAFINE (Aurobindo Pharma Limited), TERBINAFINE (Bryant Ranch Prepack), TERBINAFINE (Bryant Ranch Prepack), TERBINAFINE (Chartwell RX), TERBINAFINE (Cipla USA), TERBINAFINE (Exelan Pharmaceuticals), TERBINAFINE (NorthStar Rx LLC), TERBINAFINE (NuCare Pharmaceuticals), TERBINAFINE (PD-Rx Pharmaceuticals), TERBINAFINE (Preferred Pharmaceuticals), TERBINAFINE (Proficient Rx LP), TERBINAFINE (Proficient Rx LP), TERBINAFINE (Proficient Rx LP), TERBINAFINE (REMEDYREPACK), TERBINAFINE (REMEDYREPACK), TERBINAFINE (REMEDYREPACK), TERBINAFINE (REMEDYREPACK), TERBINAFINE (RedPharm Drug), TERBINAFINE (direct rx), TERBINAFINE HYDROCHLORIDE (A-S Medication Solutions), TERBINAFINE HYDROCHLORIDE (A-S Medication Solutions), TERBINAFINE HYDROCHLORIDE (Aphena Pharma Solutions - Tennessee), TERBINAFINE HYDROCHLORIDE (Bionpharma), TERBINAFINE HYDROCHLORIDE (Breckenridge Pharmaceutical), TERBINAFINE HYDROCHLORIDE (Bryant Ranch Prepack), TERBINAFINE HYDROCHLORIDE (Bryant Ranch Prepack), TERBINAFINE HYDROCHLORIDE (Bryant Ranch Prepack), TERBINAFINE HYDROCHLORIDE (Bryant Ranch Prepack), TERBINAFINE HYDROCHLORIDE (Cipla USA), TERBINAFINE HYDROCHLORIDE (Dr.Reddys Laboratories Limited), TERBINAFINE HYDROCHLORIDE (Golden State Medical Supply), TERBINAFINE HYDROCHLORIDE (Northwind Pharmaceuticals), TERBINAFINE HYDROCHLORIDE (NuCare Pharmaceuticals), TERBINAFINE HYDROCHLORIDE (NuCare Pharmaceuticals), TERBINAFINE HYDROCHLORIDE (PD-Rx Pharmaceuticals), TERBINAFINE HYDROCHLORIDE (Proficient Rx LP), TERBINAFINE HYDROCHLORIDE (Proficient Rx LP), TERBINAFINE HYDROCHLORIDE (Proficient Rx LP), TERBINAFINE HYDROCHLORIDE (REMEDYREPACK) |
|---|
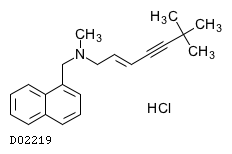
| Formula |
C21H25N. HCl
|
|---|
| Exact mass |
327.1754
|
|---|
| Mol weight |
327.89
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
|
|---|
| Remark |
| Product (DG00375): | D02219<JP/US> |
|
|---|
| Efficacy |
Antifungal, Ergosterol biosynthesis inhibitor |
|---|
| Disease |
|
|---|
| Comment |
Allylamine derivative
|
|---|
| Target |
squalene epoxidase [KO: K00511] |
|---|
| Pathway |
|
|---|
| Metabolism |
Enzyme: CYP2C9 [HSA: 1559], CYP1A2 [HSA: 1544], CYP3A4 [HSA: 1576], CYP2C8 [HSA: 1558], CYP2C19 [HSA: 1557]
|
|---|
| Interaction |
CYP inhibition: CYP2D6 [HSA: 1565]
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
D DERMATOLOGICALS
D01 ANTIFUNGALS FOR DERMATOLOGICAL USE
D01A ANTIFUNGALS FOR TOPICAL USE
D01AE Other antifungals for topical use
D01AE15 Terbinafine
D02219 Terbinafine hydrochloride (JP18/USP) <JP/US>
D01B ANTIFUNGALS FOR SYSTEMIC USE
D01BA Antifungals for systemic use
D01BA02 Terbinafine
D02219 Terbinafine hydrochloride (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Antifungals
Terbinafine
D02219 Terbinafine hydrochloride (JP18/USP)
Dermatological Agents
Topical Anti-infectives
Antifungals, Dermatological
Terbinafine
D02219 Terbinafine hydrochloride (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
26 Epidermides
265 Antiparasitic dermatosis agents
2659 Others
D02219 Terbinafine hydrochloride (JP18/USP)
6 Agents against pathologic organisms and parasites
62 Chemotherapeutics
629 Miscellaneous
6290 Miscellaneous
D02219 Terbinafine hydrochloride (JP18/USP)
Classification of Japanese OTC drugs [BR:br08313]
Agents for integumentary system
58 Ringworm drugs
D02219 Terbinafine hydrochloride (JP18/USP)
Risk category of Japanese OTC drugs [BR:br08312]
Designated second-class OTC drugs
Inorganic and organic chemicals
Terbinafine
D02219 Terbinafine hydrochloride (JP18/USP)
Second-class OTC drugs
Inorganic and organic chemicals
Terbinafine
D02219 Terbinafine hydrochloride (JP18/USP)
Drug groups [BR:br08330]
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00375 Terbinafine
D02219 Terbinafine hydrochloride
DG02918 CYP2C8 substrate
DG00375 Terbinafine
D02219 Terbinafine hydrochloride
DG01642 CYP2C9 substrate
DG00375 Terbinafine
D02219 Terbinafine hydrochloride
DG01639 CYP2C19 substrate
DG00375 Terbinafine
D02219 Terbinafine hydrochloride
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00375 Terbinafine
D02219 Terbinafine hydrochloride
Metabolizing enzyme inhibitor
DG01645 CYP2D6 inhibitor
DG00375 Terbinafine
D02219 Terbinafine hydrochloride
Antimicrobials [BR:br08307]
Antifungals
Ergosterol biosynthesis inhibitor
Other ergosterol biosynthesis inhibitor
D02219 Terbinafine hydrochloride (JP18/USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D02219 Terbinafine hydrochloride
D02219 Terbinafine hydrochloride tablets
D02219 Terbinafine hydrochloride solution
D02219 Terbinafine hydrochloride spray
D02219 Terbinafine hydrochloride cream
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D02219
Rx-to-OTC switch list in the USA [br08315.html]
D02219
Rx-to-OTC switch list in Japan [br08314.html]
D02219
Drug groups [BR:br08330]
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00375 Terbinafine
DG02918 CYP2C8 substrate
DG00375 Terbinafine
DG01642 CYP2C9 substrate
DG00375 Terbinafine
DG01639 CYP2C19 substrate
DG00375 Terbinafine
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00375 Terbinafine
Metabolizing enzyme inhibitor
DG01645 CYP2D6 inhibitor
DG00375 Terbinafine
Antimicrobials abbreviations [BR:br08327]
Antifungals
Ergosterol biosynthesis inhibitor
Other ergosterol biosynthesis inhibitor
DG00375 Terbinafine
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 23
1 X Cl 36.1001 -19.8424
2 C8x C 25.4100 -18.8300
3 C8x C 25.4100 -20.2300
4 C8x C 26.6700 -20.9300
5 C8y C 27.8600 -20.2300
6 C8y C 27.8600 -18.8300
7 C8x C 26.6700 -18.1300
8 C8x C 29.0500 -20.9300
9 C8x C 30.3100 -20.2300
10 C8x C 30.3100 -18.8300
11 C8y C 29.0500 -18.1300
12 C1b C 29.0500 -16.7300
13 N1c N 30.3100 -16.0300
14 C1b C 31.5000 -16.7300
15 C1a C 30.3100 -14.6300
16 C2b C 32.6900 -16.0300
17 C2b C 33.8800 -16.7300
18 C3b C 35.0700 -16.0300
19 C3b C 36.2600 -15.3300
20 C1d C 37.5200 -14.6300
21 C1a C 38.7100 -13.9300
22 C1a C 36.8200 -13.3700
23 C1a C 38.2200 -15.8200
BOND 23
1 2 3 2
2 3 4 1
3 4 5 2
4 5 6 1
5 6 7 2
6 2 7 1
7 5 8 1
8 8 9 2
9 9 10 1
10 10 11 2
11 6 11 1
12 11 12 1
13 12 13 1
14 13 14 1
15 13 15 1
16 14 16 1
17 16 17 2
18 17 18 1
19 18 19 3
20 19 20 1
21 20 21 1
22 20 22 1
23 20 23 1
|
|---|
|