| Entry |
|
|---|
| Name |
Lenalidomide hydrate (JAN);
Revlimid (TN) |
|---|
| Formula |
(C13H13N3O3)2. H2O
|
|---|
| Exact mass |
536.2019
|
|---|
| Mol weight |
536.54
|
|---|
| Structure |
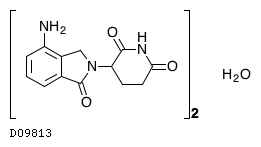
|
|---|
| Class |
Antineoplastic
DG03350 Thalidomide derivative
Anti-inflammatory
DG01985 Disease modifying anti-rheumatic drug (DMARD)
DG01936 TNF inhibitor
|
|---|
| Remark |
| Therapeutic category: | 4291 |
| Product (DG00744): | D04687<JP/US> D09813<JP> |
|
|---|
| Efficacy |
Antineoplastic, Immunomodulator, TNF-alpha inhibitor |
|---|
| Comment |
Thalidomide [DR: D00754] derivative
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04060 | Cytokine-cytokine receptor interaction |
| hsa04650 | Natural killer cell mediated cytotoxicity |
|
|---|
| Interaction |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
L ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
L04 IMMUNOSUPPRESSANTS
L04A IMMUNOSUPPRESSANTS
L04AX Other immunosuppressants
L04AX04 Lenalidomide
D09813 Lenalidomide hydrate (JAN) <JP>
USP drug classification [BR:br08302]
Antineoplastics
Immunomodulatory Agents
Imides
Lenalidomide
D09813 Lenalidomide hydrate (JAN)
Therapeutic category of drugs in Japan [BR:br08301]
4 Agents affecting cellular function
42 Antineoplastics
429 Miscellaneous
4291 Other Antitumors
D09813 Lenalidomide hydrate (JAN)
Drug groups [BR:br08330]
Antineoplastic
DG03350 Thalidomide derivative
DG00744 Lenalidomide
D09813 Lenalidomide hydrate
Anti-inflammatory
DG01985 Disease modifying anti-rheumatic drug (DMARD)
DG01936 TNF inhibitor
DG00744 Lenalidomide
D09813 Lenalidomide hydrate
Drug classes [BR:br08332]
Immunological agent
DG01936 TNF inhibitor
D09813 Lenalidomide hydrate
Antirheumatic agent
DG01985 Disease modifying anti-rheumatic drug (DMARD)
D09813 Lenalidomide hydrate
Target-based classification of drugs [BR:br08310]
Cytokines and receptors
Cytokines
Tumor necrosis factors
TNF (TNFA, TNFSF2)
D09813 Lenalidomide hydrate (JAN) <JP>
New drug approvals in Europe [br08329.html]
European public assessment reports (EPAR) authorised medicine
D09813
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D09813
Drug groups [BR:br08330]
Antineoplastic
DG03350 Thalidomide derivative
DG00744 Lenalidomide
Anti-inflammatory
DG01985 Disease modifying anti-rheumatic drug (DMARD)
DG01936 TNF inhibitor
DG00744 Lenalidomide
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 39
1 C8x C 19.9500 -19.5300
2 C8x C 19.9500 -20.9300
3 C8x C 21.2100 -21.6300
4 C8y C 22.4000 -20.9300
5 C8y C 22.4000 -19.5300
6 C8y C 21.2100 -18.8300
7 C5x C 23.7300 -21.4200
8 N1y N 24.5700 -20.2300
9 C1x C 23.7300 -19.1100
10 C1y C 25.9000 -20.2300
11 O5x O 24.1500 -22.7500
12 N1a N 21.2100 -17.4300
13 C1x C 26.6700 -21.4900
14 C1x C 28.0700 -21.4900
15 C5x C 28.7700 -20.2300
16 N1x N 28.0700 -19.0400
17 C5x C 26.6700 -19.0400
18 O5x O 25.9700 -17.8500
19 O5x O 30.1700 -20.2300
20 O0 O 35.2800 -20.7900
21 C8x C 19.9500 -19.5300
22 C8x C 19.9500 -20.9300
23 C8x C 21.2100 -21.6300
24 C8y C 22.4000 -20.9300
25 C8y C 22.4000 -19.5300
26 C8y C 21.2100 -18.8300
27 N1a N 21.2100 -17.4300
28 C1x C 23.7300 -19.1100
29 N1y N 24.5700 -20.2300
30 C5x C 23.7300 -21.4200
31 O5x O 24.1500 -22.7500
32 C1y C 25.9000 -20.2300
33 C1x C 26.6700 -21.4900
34 C1x C 28.0700 -21.4900
35 C5x C 28.7700 -20.2300
36 N1x N 28.0700 -19.0400
37 C5x C 26.6700 -19.0400
38 O5x O 25.9700 -17.8500
39 O5x O 30.1700 -20.2300
BOND 42
1 1 2 2
2 2 3 1
3 3 4 2
4 4 5 1
5 5 6 2
6 1 6 1
7 4 7 1
8 7 8 1
9 8 9 1
10 5 9 1
11 8 10 1
12 7 11 2
13 6 12 1
14 10 13 1
15 13 14 1
16 14 15 1
17 15 16 1
18 16 17 1
19 10 17 1
20 17 18 2
21 15 19 2
22 21 22 2
23 22 23 1
24 23 24 2
25 24 25 1
26 25 26 2
27 21 26 1
28 24 30 1
29 30 29 1
30 29 28 1
31 25 28 1
32 29 32 1
33 30 31 2
34 26 27 1
35 32 33 1
36 33 34 1
37 34 35 1
38 35 36 1
39 36 37 1
40 32 37 1
41 37 38 2
42 35 39 2
BRACKET 1 19.1800 -23.8700 19.1800 -16.3100
1 31.0100 -16.3100 31.0100 -23.8700
1 2
ORIGINAL 1 1 2 3 4 5 6 12 9 8 7 11 10 13 14 15 16
1 17 18 19
REPEAT 1 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
1 37 38 39
|
|---|