 | | DRUG: ロラゼパム | |
| エントリ |
|
|---|
| 一般名 |
ロラゼパム (JP18);
Lorazepam (JP18/USP/INN) |
|---|
| 商品名 |
|
|---|
| 後発品 |
|
|---|
| 米国の商品 |
|
|---|
| 後発品 |
LORAZEPAM (A-S Medication Solutions), LORAZEPAM (A-S Medication Solutions), LORAZEPAM (A-S Medication Solutions), LORAZEPAM (A-S Medication Solutions), LORAZEPAM (ANI Pharmaceuticals), LORAZEPAM (Advanced Rx of Tennessee), LORAZEPAM (American Health Packaging), LORAZEPAM (Amneal Pharmaceuticals LLC), LORAZEPAM (Amneal Pharmaceuticals LLC), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Aphena Pharma Solutions - Tennessee), LORAZEPAM (Asclemed USA), LORAZEPAM (Asclemed USA), LORAZEPAM (Aurolife Pharma LLC), LORAZEPAM (Bryant Ranch Prepack), LORAZEPAM (Bryant Ranch Prepack), LORAZEPAM (Bryant Ranch Prepack), LORAZEPAM (Bryant Ranch Prepack), LORAZEPAM (Bryant Ranch Prepack), LORAZEPAM (Bryant Ranch Prepack), LORAZEPAM (Cardinal Health 107), LORAZEPAM (Chartwell RX), LORAZEPAM (Chartwell RX), LORAZEPAM (Clinical Solutions Wholesale), LORAZEPAM (DIRECT RX), LORAZEPAM (DirectRx), LORAZEPAM (Direct_Rx), LORAZEPAM (Fresenius Kabi USA), LORAZEPAM (Golden State Medical Supply), LORAZEPAM (Hikma Pharmaceuticals USA), LORAZEPAM (Hospira), LORAZEPAM (International Medication Systems), LORAZEPAM (Leading Pharma), LORAZEPAM (Major Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (NuCare Pharmaceuticals), LORAZEPAM (PD-Rx Pharmaceuticals), LORAZEPAM (PD-Rx Pharmaceuticals), LORAZEPAM (PD-Rx Pharmaceuticals), LORAZEPAM (PD-Rx Pharmaceuticals), LORAZEPAM (PD-Rx Pharmaceuticals), LORAZEPAM (PD-Rx Pharmaceuticals), LORAZEPAM (PharmPak), LORAZEPAM (Pharmaceutical Associates), LORAZEPAM (Preferred Pharmaceuticals), LORAZEPAM (Preferred Pharmaceuticals), LORAZEPAM (Preferred Pharmaceuticals), LORAZEPAM (Proficient Rx LP), LORAZEPAM (Proficient Rx LP), LORAZEPAM (Proficient Rx LP), LORAZEPAM (Proficient Rx LP), LORAZEPAM (Proficient Rx LP), LORAZEPAM (Proficient Rx LP), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (REMEDYREPACK), LORAZEPAM (RedPharm Drug), LORAZEPAM (RedPharm Drug), LORAZEPAM (RedPharm Drug), LORAZEPAM (RedPharm Drug), LORAZEPAM (RedPharm Drug), LORAZEPAM (RedPharm Drug), LORAZEPAM (RedPharm Drug), LORAZEPAM (SUN PHARMACEUTICAL INDUSTRIES), LORAZEPAM (Teva Pharmaceuticals USA) |
|---|
| 組成式 |
C15H10Cl2N2O2
|
|---|
| 質量 |
320.0119
|
|---|
| 分子量 |
321.15
|
|---|
| 構造式 |
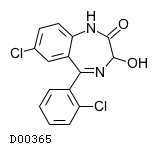
|
|---|
| Simcomp |
|
|---|
| クラス |
|
|---|
| コード |
|
|---|
| 効能 |
抗不安薬, 抗けいれん薬, マイナートランキライザー |
|---|
| 疾患 |
|
|---|
| コメント |
ベンゾジアゼピン誘導体
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 代謝 |
Enzyme: UGT2B7 [HSA: 7364], UGT2B15 [HSA: 7366]
|
|---|
| 相互作用 |
|
|---|
| 構造マップ |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
1 神経系及び感覚器官用医薬品
11 中枢神経系用薬
112 催眠鎮静剤、抗不安剤
1124 ベンゾジアゼピン系製剤
D00365 ロラゼパム (JP18)
113 抗てんかん剤
1139 その他の抗てんかん剤
D00365 ロラゼパム (JP18)
医療用医薬品のATC分類 [BR:jp08303]
N 神経系
N05 精神抑制薬
N05B 抗不安薬
N05BA ベンゾジアゼピン誘導体
N05BA06 ロラゼパム
D00365 ロラゼパム (JP18) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
抗けいれん薬
ガンマアミノ酪酸(GABA)増強剤
ベンゾジアゼピン
ロラゼパム
D00365 ロラゼパム (JP18)
抗不安薬
ベンゾジアゼピン
ロラゼパム
D00365 ロラゼパム (JP18)
医薬品グループ [BR:jp08330]
精神神経系用薬
DG01567 GABA-A受容体作動薬
D00365 ロラゼパム
DG01914 ベンゾジアゼピン系抗不安薬
D00365 ロラゼパム
DG03199 抗てんかん薬
D00365 ロラゼパム
代謝酵素基質薬
DG02924 UGT基質薬
DG03190 UGT2B7基質薬
D00365 ロラゼパム
DG03187 UGT2B15基質薬
D00365 ロラゼパム
医薬品クラス [BR:jp08332]
精神神経系用薬
DG03199 抗てんかん薬 [商品一覧]
D00365 ロラゼパム
ターゲットに基づく医薬品分類 [BR:jp08310]
イオンチャネル
リガンド依存性イオンチャネル
GABA (イオンチャネル型)
GABR
D00365 ロラゼパム (JP18) <JP/US>
日本薬局方収載医薬品 [BR:jp08311]
化学薬品等
D00365 ロラゼパム
薬物代謝酵素とトランスポーター [jp08309.html]
薬物代謝酵素
D00365
麻薬と向精神薬 [jp08308.html]
麻薬、麻薬原料植物、向精神薬及び麻薬向精神薬原料を指定する政令 第3条 による向精神薬 (78物質)
D00365
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|