| Entry |
|
|---|
| Name |
Phenylpropanoid biosynthesis |
|---|
| Description |
Phenylpropanoids are a group of plant secondary metabolites derived from phenylalanine and having a wide variety of functions both as structural and signaling molecules. Phenylalanine is first converted to cinnamic acid by deamination. It is followed by hydroxylation and frequent methylation to generate coumaric acid and other acids with a phenylpropane (C6-C3) unit. Reduction of the CoA-activated carboxyl groups of these acids results in the corresponding aldehydes and alcohols. The alcohols are called monolignols, the starting compounds for biosynthesis of lignin.
|
|---|
| Class |
Metabolism; Biosynthesis of other secondary metabolites
|
|---|
| Pathway map |
| rn00940 | Phenylpropanoid biosynthesis |
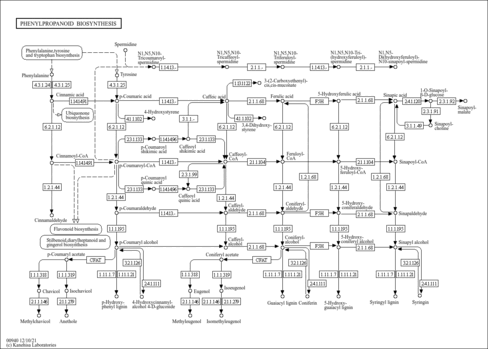
|
|---|
| Module |
| M00039 | Monolignol biosynthesis, phenylalanine/tyrosine => monolignol [PATH:rn00940] |
| M00137 | Flavanone biosynthesis, phenylalanine => naringenin [PATH:rn00940] |
|
|---|
| Other DBs |
|
|---|
| Reaction |
| R00697 | L-phenylalanine ammonia-lyase (trans-cinnamate-forming) |
| R00737 | L-tyrosine ammonia-lyase (trans-p-hydroxycinnamate-forming) |
| R01615 | 4-hydroxycinnamyl aldehyde:NADP+ oxidoreductase (CoA-hydroxycinnamoylating) |
| R01616 | 4-coumarate:CoA ligase (AMP-forming) |
| R01941 | caffeic aldehyde:NADP+ oxidoreductase (CoA-caffeoylating) |
| R01942 | S-adenosyl-L-methionine:caffeoyl-CoA 3-O-methyltransferase |
| R01943 | caffeate:CoA ligase (AMP-forming); 3,4-dihydroxy-trans-cinnamate:CoA ligase (AMP-forming) |
| R01945 | caffeoyl-CoA:quinate O-(3,4-dihydroxycinnamoyl)transferase |
| R02193 | coniferyl aldehyde:NADP+ oxidoreductase (CoA-feruloylating) |
| R02194 | ferulate:CoA ligase (AMP-forming) |
| R02220 | sinapoyl aldehyde:NADP+ oxidoreductase (CoA-sinapoylating) |
| R02221 | sinapate:CoA ligase (AMP-forming) |
| R02253 | trans-cinnamate,[reduced NADPH---hemoprotein reductase]:oxygen oxidoreductase (4-hydroxylating) |
| R02255 | trans-cinnamate:CoA ligase (AMP-forming) |
| R02379 | S-adenosyl-L-methionine:3,4-dihydroxy-trans-cinnamate 3-O-methyltransferase |
| R02380 | UDPglucose:sinapate D-glucosyltransferase |
| R02381 | sinapoylcholine sinapohydrolase |
| R02416 | 4-coumaroyl-CoA:shikimate O-(hydroxycinnamoyl)transferase |
| R02506 | cinnamaldehyde:NADP+ oxidoreductase (CoA-cinnamoylating) |
| R02593 | coniferyl alcohol:NADP+ oxidoreductase |
| R02594 | UDPglucose:coniferyl-alcohol 4'-beta-D-glucosyltransferase |
| R02595 | coniferin beta-D-glucosidase |
| R02596 | donor:hydrogen-peroxide oxidoreductase |
| R02952 | phenylacrylic acid decarboxylase |
| R03075 | 1-O-(4-hydroxy-3,5-dimethoxycinnamoyl)-beta-D-glucose:choline 1-O-(4-hydroxy-3,5-dimethoxycinnamoyl)transferase |
| R03323 | 1-O-sinapoyl-beta-D-glucose:(S)-malate O-sinapoyltransferase |
| R03365 | 3,4-dihydroxy-trans-cinnamate:oxygen 3,4-oxidoreductase (decyclizing) |
| R03366 | S-adenosyl-L-methionine:3,4-dihydroxy-trans-cinnamate 3-O-methyltransferase |
| R03604 | syringin beta-D-glucosidase |
| R03605 | UDPglucose:coniferyl-alcohol 4'-beta-D-glucosyltransferase |
| R03918 | sinapyl alcohol:NADP+ oxidoreductase |
| R03919 | donor:hydrogen-peroxide oxidoreductase |
| R04005 | UDPglucose:coniferyl-alcohol 4'-beta-D-glucosyltransferase |
| R04006 | 4-hydroxycinnamyl alcohol 4-D-glucoside beta-D-glucosidase |
| R04007 | donor:hydrogen-peroxide oxidoreductase |
| R04342 | trans-5-O-(4-coumaroyl)-D-quinate,[reduced NADPH---hemoprotein reductase]:oxygen oxidoreductase (3'-hydroxylating) |
| R05700 | coniferyl aldehyde:NAD oxidoreductase |
| R05701 | coniferyl aldehyde:NADP oxidoreductase |
| R06569 | cinnamaldehyde:NADP+ oxidoreductase (CoA-cinnamoylating) |
| R06570 | cinnamyl-alcohol:NADP+ oxidoreductase |
| R06571 | cinnamyl-alcohol:NADP+ oxidoreductase |
| R06574 | caffeic aldehyde:3,4-dihydroxy-trans-cinnamate 3-O-methyltransferase |
| R06575 | S-adenosyl-L-methionine:3,4-dihydroxy-trans-cinnamate 3-O-methyltransferase |
| R06576 | S-adenosyl-L-methionine:3,4-dihydroxy-trans-cinnamate 3-O-methyltransferase |
| R06577 | S-adenosyl-L-methionine:3,4-dihydroxy-trans-cinnamate 3-O-methyltransferase |
| R06578 | S-adenosyl-L-methionine:caffeoyl-CoA 3-O-methyltransferase |
| R06582 | trans-5-O-(4-coumaroyl)-D-shikimate,[reduced NADPH---hemoprotein reductase]:oxygen oxidoreductase (3'-hydroxylating) |
| R06583 | 4-coumarate:CoA ligase (AMP-forming) |
| R07240 | S-adenosyl-L-methionine:isoeugenol O-methyltransferase |
| R07432 | p-coumaroyl-CoA:quinate hydroxycinnamoyltransferase |
| R07433 | 5-O-caffeoylshikimic acid:shikimate hydroxycinnamoyltransferase |
| R07436 | p-coumaroyl-CoA:caffeoyl-CoA 3-hydroxylase |
| R07437 | 4-coumaryl alcohol:NADP+ oxidoreductase |
| R07441 | sinapoyl aldehyde:NAD oxidoreductase |
| R07442 | sinapoyl aldehyde:NADP oxidoreductase |
| R07443 | donor:hydrogen-peroxide oxidoreductase |
| R07826 | p-coumarate 3-hydroxylase |
| R08815 | cinnamoyl-CoA,[reduced NADPH---hemoprotein reductase]:oxygen oxidoreductase (4-hydroxylating) |
| R09931 | eugenol:NADP+ oxidoreductase (coniferyl acetate reducing) |
| R10248 | eugenol:NADP+ oxidoreductase (coniferyl ester reducing) |
| R10249 | chavicol:NADP+ oxidoreductase |
| R10250 | S-adenosyl-L-methionine:eugenol O-methyltransferase |
| R10251 | S-adenosyl-L-methionine:chavicol O-methyltransferase |
| R10252 | S-adenosyl-L-methionine:trans-anol O-methyltransferase |
| R10289 | isochavicol:NADP+ oxidoreductase |
|
|---|
| Compound |
| C01175 | 1-O-Sinapoyl-beta-D-glucose |
| C04366 | 3-(2-Carboxyethenyl)-cis,cis-muconate |
| C05608 | 4-Hydroxycinnamyl aldehyde |
| C05855 | 4-Hydroxycinnamyl alcohol 4-D-glucoside |
| C10434 | 5-O-Caffeoylshikimic acid |
| C12204 | 5-Hydroxyconiferaldehyde |
| C12205 | 5-Hydroxyconiferyl alcohol |
| C12208 | p-Coumaroyl quinic acid |
| C15807 | 5-Hydroxy-guaiacyl lignin |
| C18069 | N1,N5,N10-Tricoumaroyl spermidine |
| C18070 | N1,N5,N10-Tricaffeoyl spermidine |
| C18071 | N1,N5,N10-Triferuloyl spermidine |
| C18072 | N1,N5,N10-Tri(hydroxyferuloyl) spermidine |
| C18073 | N1,N5-Di(hydroxyferuloyl)-N10-sinapoyl spermidine |
|
|---|
| Reference |
|
|---|
| Authors |
Nair RB, Bastress KL, Ruegger MO, Denault JW, Chapple C. |
|---|
| Title |
The Arabidopsis thaliana REDUCED EPIDERMAL FLUORESCENCE1 gene encodes an aldehyde dehydrogenase involved in ferulic acid and sinapic acid biosynthesis. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Baucher M, Halpin C, Petit-Conil M, Boerjan W. |
|---|
| Title |
Lignin: genetic engineering and impact on pulping. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Rogers LA, Dubos C, Cullis IF, Surman C, Poole M, Willment J, Mansfield SD, Campbell MM. |
|---|
| Title |
Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Whetten R, Sederoff R. |
|---|
| Title |
Lignin Biosynthesis. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Lorenzen M, Racicot V, Strack D, Chapple C. |
|---|
| Title |
Sinapic acid ester metabolism in wild type and a sinapoylglucose-accumulating mutant of arabidopsis. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Chapple CC, Vogt T, Ellis BE, Somerville CR. |
|---|
| Title |
An Arabidopsis mutant defective in the general phenylpropanoid pathway. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Raes J, Rohde A, Christensen JH, Van de Peer Y, Boerjan W. |
|---|
| Title |
Genome-wide characterization of the lignification toolbox in Arabidopsis. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Costa MA, Collins RE, Anterola AM, Cochrane FC, Davin LB, Lewis NG. |
|---|
| Title |
An in silico assessment of gene function and organization of the phenylpropanoid pathway metabolic networks in Arabidopsis thaliana and limitations thereof. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Meyermans H, Morreel K, Lapierre C, Pollet B, De Bruyn A, Busson R, Herdewijn P, Devreese B, Van Beeumen J, Marita JM, Ralph J, Chen C, Burggraeve B, Van Montagu M, Messens E, Boerjan W. |
|---|
| Title |
Modifications in lignin and accumulation of phenolic glucosides in poplar xylem upon down-regulation of caffeoyl-coenzyme A O-methyltransferase, an enzyme involved in lignin biosynthesis. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Gachon CM, Langlois-Meurinne M, Henry Y, Saindrenan P. |
|---|
| Title |
Transcriptional co-regulation of secondary metabolism enzymes in Arabidopsis: functional and evolutionary implications. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Li L, Popko JL, Umezawa T, Chiang VL. |
|---|
| Title |
5-hydroxyconiferyl aldehyde modulates enzymatic methylation for syringyl monolignol formation, a new view of monolignol biosynthesis in angiosperms. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Yamauchi K, Yasuda S, Fukushima K. |
|---|
| Title |
Evidence for the biosynthetic pathway from sinapic acid to syringyl lignin using labeled sinapic acid with stable isotope at both methoxy groups in Robinia pseudoacacia and Nerium indicum. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Eckardt NA. |
|---|
| Title |
Probing the mysteries of lignin biosynthesis: the crystal structure of caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase provides new insights. |
|---|
| Journal |
|
|---|
Related
pathway |
| rn00130 | Ubiquinone and other terpenoid-quinone biosynthesis |
| rn00400 | Phenylalanine, tyrosine and tryptophan biosynthesis |
| rn00945 | Stilbenoid, diarylheptanoid and gingerol biosynthesis |
|
|---|
| KO pathway |
|
|---|