| Entry |
|
|---|
| Name |
Lansoprazole (JP18/USP/INN);
Prevacid (TN) |
|---|
| Product |
|
|---|
| Generic |
LANSOPRAZOLE (A-S Medication Solutions), LANSOPRAZOLE (A-S Medication Solutions), LANSOPRAZOLE (A-S Medication Solutions), LANSOPRAZOLE (A-S Medication Solutions), LANSOPRAZOLE (A-S Medication Solutions), LANSOPRAZOLE (Advanced Rx Pharmacy of Tennessee), LANSOPRAZOLE (Advanced Rx Pharmacy of Tennessee), LANSOPRAZOLE (Ajanta Pharma USA), LANSOPRAZOLE (American Health Packaging), LANSOPRAZOLE (Ascend Laboratories), LANSOPRAZOLE (Asclemed USA), LANSOPRAZOLE (Aurobindo Pharma Limited), LANSOPRAZOLE (BluePoint Laboratories), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Bryant Ranch Prepack), LANSOPRAZOLE (Camber Pharmaceuticals), LANSOPRAZOLE (Cardinal Health 107), LANSOPRAZOLE (Dr.Reddy's Laboratories), LANSOPRAZOLE (Dr.Reddys Laboratories), LANSOPRAZOLE (Golden State Medical Supply), LANSOPRAZOLE (Lifestar Pharma LLC.), LANSOPRAZOLE (Macleods Pharmaceuticals Limited), LANSOPRAZOLE (Major Pharmaceuticals), LANSOPRAZOLE (Mylan Institutional), LANSOPRAZOLE (Mylan Pharmaceuticals), LANSOPRAZOLE (Mylan Pharmaceuticals), LANSOPRAZOLE (NorthStar Rx LLC), LANSOPRAZOLE (NorthStar Rx LLC), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (NuCare Pharmaceuticals), LANSOPRAZOLE (Preferred Pharmaceuticals), LANSOPRAZOLE (Preferred Pharmaceuticals), LANSOPRAZOLE (Preferred Pharmaceuticals), LANSOPRAZOLE (Proficient Rx LP), LANSOPRAZOLE (Proficient Rx LP), LANSOPRAZOLE (Quallent Pharmaceuticals Health LLC), LANSOPRAZOLE (Rising Pharma Holdings), LANSOPRAZOLE (Teva Pharmaceuticals USA), LANSOPRAZOLE (Teva Pharmaceuticals USA), LANSOPRAZOLE (Xiromed LLC), LANSOPRAZOLE (Zydus Lifesciences Limited), LANSOPRAZOLE (Zydus Pharmaceuticals USA), LANSOPRAZOLE DR (Direct_Rx) |
|---|
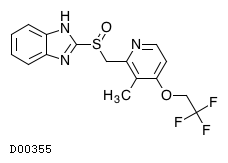
| Formula |
C16H14F3N3O2S
|
|---|
| Exact mass |
369.0759
|
|---|
| Mol weight |
369.36
|
|---|
| Structure |
|
|---|
| Simcomp |
|
|---|
| Class |
Gastrointestinal agent
DG01975 Agents for peptic ulcer
DG01646 Proton pump inhibitor (PPI)
Metabolizing enzyme substrate
DG01639 CYP2C19 substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
Metabolizing enzyme inhibitor
DG01933 CYP2C19 inhibitor
Transporter inhibitor
DG02867 ABCC2 inhibitor
|
|---|
| Remark |
| Therapeutic category: | 2329 |
| Product (mixture): | D08774<US> D10527<JP> |
|
|---|
| Efficacy |
Anti-ulcerative, Proton pump inhibitor |
|---|
| Disease |
Duodenal ulcer [DS: H01634] H. pylori eradication [DS: H00320] Gastric ulcer [DS: H01634] Gastroesophageal reflux disease [DS: H01602] Zollinger-Ellison syndrome [DS: H01522] |
|---|
| Comment |
Benzimidazole derivative
|
|---|
| Target |
|
|---|
| Pathway |
|
|---|
| Metabolism |
Enzyme: CYP2C19 [HSA: 1557], CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
CYP inhibition: CYP2C19 [HSA: 1557]
Transporter inhibition: ABCC2 [HSA: 1244]
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
A ALIMENTARY TRACT AND METABOLISM
A02 DRUGS FOR ACID RELATED DISORDERS
A02B DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
A02BC Proton pump inhibitors
A02BC03 Lansoprazole
D00355 Lansoprazole (JP18/USP/INN) <JP/US>
USP drug classification [BR:br08302]
Gastrointestinal Agents
Proton Pump Inhibitors
Lansoprazole
D00355 Lansoprazole (JP18/USP/INN)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
23 Digestive organ agents
232 Peptic ulcer agents
2329 Others
D00355 Lansoprazole (JP18/USP/INN)
Risk category of Japanese OTC drugs [BR:br08312]
Drugs requiring guidance
New approved switch OTC drugs
Lansoprazole
D00355 Lansoprazole (JP18/USP/INN)
Drug groups [BR:br08330]
Gastrointestinal agent
DG01975 Agents for peptic ulcer
DG01646 Proton pump inhibitor (PPI)
D00355 Lansoprazole
Metabolizing enzyme substrate
DG01639 CYP2C19 substrate
D00355 Lansoprazole
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
D00355 Lansoprazole
Metabolizing enzyme inhibitor
DG01933 CYP2C19 inhibitor
D00355 Lansoprazole
Transporter inhibitor
DG02867 ABCC2 inhibitor
D00355 Lansoprazole
Drug classes [BR:br08332]
Gastrointestinal agent
DG01646 Proton pump inhibitor (PPI)
D00355 Lansoprazole
Target-based classification of drugs [BR:br08310]
Enzymes
Hydrolases (EC3)
ATPase
ATP4
D00355 Lansoprazole (JP18/USP/INN) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00355 Lansoprazole
D00355 Lansoprazole delayed-release orally disintegration tablets
D00355 Lansoprazole delayed-release capsules
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00355
Drug transporters
D00355
Pharmacogenomic biomarkers [br08341.html]
Polymorphisms and mutations affecting drug response
D00355
Rx-to-OTC switch list in the USA [br08315.html]
D00355
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 25
1 C8x C 14.2800 -19.3900
2 C8x C 14.2800 -20.7900
3 C8x C 15.4924 -21.4900
4 C8y C 16.7049 -20.7900
5 C8y C 16.7049 -19.3900
6 C8x C 15.4924 -18.6900
7 N5x N 18.0364 -21.2226
8 C8y C 18.8593 -20.0900
9 N4x N 18.0364 -18.9574
10 S4a S 20.2300 -20.0900
11 C1b C 20.9300 -21.3024
12 O3c O 20.9300 -18.8776
13 C8y C 22.3298 -21.3024
14 C8y C 23.0203 -22.4980
15 C8y C 24.4203 -22.4978
16 C8x C 25.1201 -21.2853
17 C8x C 24.4297 -20.0898
18 N5x N 23.0297 -20.0899
19 C1a C 22.3349 -23.6852
20 O2a O 25.1110 -23.6932
21 C1b C 26.5296 -23.6932
22 C1d C 27.2156 -24.8806
23 X F 28.4280 -24.1806
24 X F 26.0032 -25.5806
25 X F 27.9156 -26.0931
BOND 27
1 1 2 2
2 2 3 1
3 3 4 2
4 4 5 1
5 5 6 2
6 1 6 1
7 4 7 1
8 7 8 2
9 8 9 1
10 5 9 1
11 8 10 1
12 10 11 1
13 10 12 2
14 11 13 1
15 13 14 2
16 14 15 1
17 15 16 2
18 16 17 1
19 17 18 2
20 13 18 1
21 14 19 1
22 15 20 1
23 20 21 1
24 21 22 1
25 22 23 1
26 22 24 1
27 22 25 1
|
|---|
|