 | | DRUG: Diltiazem hydrochloride | |
| Entry |
|
|---|
| Name |
Diltiazem hydrochloride (JP18/USP);
Cardizem (TN);
Cartia XT (TN);
Dilacor XR (TN);
Dilt-CD (TN) |
|---|
| Product |
|
|---|
| Generic |
CARTIA (Actavis Pharma), CARTIA (Bryant Ranch Prepack), CARTIA (Bryant Ranch Prepack), CARTIA XT (DirectRx), DILTIAZEM HCI (HF Acquisition Co LLC), DILTIAZEM HCI (HF Acquisition Co LLC), DILTIAZEM HCL ER (Direct_Rx), DILTIAZEM HYDROCHLORIDE (A-S Medication Solutions), DILTIAZEM HYDROCHLORIDE (A-S Medication Solutions), DILTIAZEM HYDROCHLORIDE (A-S Medication Solutions), DILTIAZEM HYDROCHLORIDE (A-S Medication Solutions), DILTIAZEM HYDROCHLORIDE (A-S Medication Solutions), DILTIAZEM HYDROCHLORIDE (A-S Medication Solutions), DILTIAZEM HYDROCHLORIDE (A-S Medication Solutions), DILTIAZEM HYDROCHLORIDE (Accord Healthcare), DILTIAZEM HYDROCHLORIDE (Actavis Pharma), DILTIAZEM HYDROCHLORIDE (Alembic Pharmaceuticals Limited), DILTIAZEM HYDROCHLORIDE (Alembic Pharmaceuticals Limited), DILTIAZEM HYDROCHLORIDE (Alembic Pharmaceuticals Limited), DILTIAZEM HYDROCHLORIDE (Alembic Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Alembic Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Alembic Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (American Health Packaging), DILTIAZEM HYDROCHLORIDE (American Health Packaging), DILTIAZEM HYDROCHLORIDE (Apotex Corp.), DILTIAZEM HYDROCHLORIDE (Athenex Pharmaceutical Division), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Bryant Ranch Prepack), DILTIAZEM HYDROCHLORIDE (Cardinal Health 107), DILTIAZEM HYDROCHLORIDE (Chartwell RX), DILTIAZEM HYDROCHLORIDE (Cipla USA), DILTIAZEM HYDROCHLORIDE (Coupler LLC), DILTIAZEM HYDROCHLORIDE (Coupler LLC), DILTIAZEM HYDROCHLORIDE (Coupler LLC), DILTIAZEM HYDROCHLORIDE (Coupler LLC), DILTIAZEM HYDROCHLORIDE (Edenbridge Pharmaceuticals LLC.), DILTIAZEM HYDROCHLORIDE (Endo USA), DILTIAZEM HYDROCHLORIDE (Eugia US LLC), DILTIAZEM HYDROCHLORIDE (GLENMARK PHARMACEUTICALS), DILTIAZEM HYDROCHLORIDE (Golden State Medical Supply), DILTIAZEM HYDROCHLORIDE (Hikma Pharmaceuticals USA), DILTIAZEM HYDROCHLORIDE (Hikma Pharmaceuticals USA), DILTIAZEM HYDROCHLORIDE (Hospira), DILTIAZEM HYDROCHLORIDE (Ingenus Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Ingenus Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Major Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (McKesson Corporation dba SKY Packaging), DILTIAZEM HYDROCHLORIDE (Medical Purchasing Solutions), DILTIAZEM HYDROCHLORIDE (Medical Purchasing Solutions), DILTIAZEM HYDROCHLORIDE (Mylan Institutional), DILTIAZEM HYDROCHLORIDE (Mylan Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (NCS HealthCare of KY), DILTIAZEM HYDROCHLORIDE (NCS HealthCare of KY), DILTIAZEM HYDROCHLORIDE (NORTHSTAR RX LLC), DILTIAZEM HYDROCHLORIDE (Northstar Rx LLC), DILTIAZEM HYDROCHLORIDE (Northwind Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Northwind Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Northwind Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (NuCare Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (NuCare Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (NuCare Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (OCEANSIDE PHARMACEUTICALS), DILTIAZEM HYDROCHLORIDE (Oceanside Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Oceanside Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Oceanside Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Preferred Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (ProPharma Distribution), DILTIAZEM HYDROCHLORIDE (Proficient Rx LP), DILTIAZEM HYDROCHLORIDE (REMEDYREPACK), DILTIAZEM HYDROCHLORIDE (Sagent Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (ScieGen Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (ScieGen Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Slate Run Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Slate Run Pharmaceuticals), DILTIAZEM HYDROCHLORIDE (Sun Pharmaceutical Industries), DILTIAZEM HYDROCHLORIDE (Sun Pharmaceutical Industries), DILTIAZEM HYDROCHLORIDE (Sun Pharmaceutical Industries), DILTIAZEM HYDROCHLORIDE (Teva Pharmaceuticals USA), DILTIAZEM HYDROCHLORIDE (Upsher-Smith Laboratories), DILTIAZEM HYDROCHLORIDE (Upsher-Smith Laboratories), DILTIAZEM HYDROCHLORIDE (Zydus Lifesciences Limited), DILTIAZEM HYDROCHLORIDE (Zydus Pharmaceuticals (USA)), DILTIAZEM HYDROCHLORIDE EXTENDED RELEASE (direct rx), DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE (AvKARE), DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE (AvKARE), DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE (Dr. Reddy's Labratories), DILTIAZEM HYDROCHLORIDE EXTENDED-RELEASE (Mayne Pharma), MATZIM LA (Actavis Pharma), TIADYLT ER (Bryant Ranch Prepack), TIADYLT ER (Preferred Pharmaceuticals), TIADYLT ER (Zydus Lifesciences Limited), TIADYLT ER (Zydus Pharmaceuticals (USA)) |
|---|
| Formula |
C22H26N2O4S. HCl
|
|---|
| Exact mass |
450.1380
|
|---|
| Mol weight |
450.98
|
|---|
| Structure |
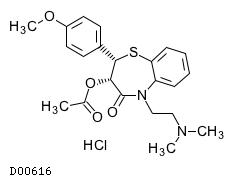
|
|---|
| Simcomp |
|
|---|
| Class |
Cardiovascular agent
DG01575 Calcium channel blocker
DG01496 Calcium channel L type blocker
DG01653 Antiarrhythmics
DG03231 Antihypertensive
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
|
|---|
| Remark |
| Therapeutic category: | 2171 |
| Product (DG00331): | D00616<JP/US> |
|
|---|
| Efficacy |
Antiarrhythmic, Antihypertensive, Vasodilator (coronary), Calcium channel blocker |
|---|
| Disease |
|
|---|
| Comment |
Benzodiazepine derivative
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04261 | Adrenergic signaling in cardiomyocytes |
| hsa04270 | Vascular smooth muscle contraction |
|
|---|
| Metabolism |
Enzyme: CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
C CARDIOVASCULAR SYSTEM
C05 VASOPROTECTIVES
C05A AGENTS FOR TREATMENT OF HEMORRHOIDS AND ANAL FISSURES FOR TOPICAL USE
C05AE Muscle relaxants
C05AE03 Diltiazem
D00616 Diltiazem hydrochloride (JP18/USP) <JP/US>
C08 CALCIUM CHANNEL BLOCKERS
C08D SELECTIVE CALCIUM CHANNEL BLOCKERS WITH DIRECT CARDIAC EFFECTS
C08DB Benzothiazepine derivatives
C08DB01 Diltiazem
D00616 Diltiazem hydrochloride (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Cardiovascular Agents
Antiarrhythmics
Vaughn Williams-Class IV
Diltiazem
D00616 Diltiazem hydrochloride (JP18/USP)
Calcium Channel Blocking Agents, Nondihydropyridines
Diltiazem
D00616 Diltiazem hydrochloride (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
21 Cardiovascular agents
217 Vasodilators
2171 Coronary dilators
D00616 Diltiazem hydrochloride (JP18/USP)
Drug groups [BR:br08330]
Cardiovascular agent
DG01575 Calcium channel blocker
DG01496 Calcium channel L type blocker
DG00331 Diltiazem
D00616 Diltiazem hydrochloride
DG01653 Antiarrhythmics
DG00331 Diltiazem
D00616 Diltiazem hydrochloride
DG03231 Antihypertensive
DG00331 Diltiazem
D00616 Diltiazem hydrochloride
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00331 Diltiazem
D00616 Diltiazem hydrochloride
Drug classes [BR:br08332]
Cardiovascular agent
DG03231 Antihypertensive
D00616 Diltiazem hydrochloride
DG01653 Antiarrhythmics
D00616 Diltiazem hydrochloride
Target-based classification of drugs [BR:br08310]
Ion channels
Voltage-gated ion channels
Calcium channels
CACNA1-L
D00616 Diltiazem hydrochloride (JP18/USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00616 Diltiazem hydrochloride
D00616 Diltiazem hydrochloride extended-release capsules
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00616
Drug groups [BR:br08330]
Cardiovascular agent
DG01575 Calcium channel blocker
DG01496 Calcium channel L type blocker
DG00331 Diltiazem
DG01653 Antiarrhythmics
DG00331 Diltiazem
DG03231 Antihypertensive
DG00331 Diltiazem
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00331 Diltiazem
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 30
1 C5x C 26.2434 -16.0394
2 C1y C 25.4013 -14.9298
3 C1y C 25.7365 -13.5730
4 S2x S 26.9975 -12.9949
5 N1y N 27.6492 -16.0646
6 C8y C 28.2511 -13.6172
7 C8y C 28.5376 -14.9859
8 C8x C 29.8688 -15.4230
9 C8x C 30.9130 -14.4886
10 C8x C 30.6266 -13.1199
11 C8x C 29.2952 -12.6828
12 O5x O 25.5217 -17.2390
13 C1b C 28.3273 -17.2894
14 C8y C 24.7294 -12.6046
15 C8x C 24.7294 -11.2046
16 C8x C 23.5170 -10.5046
17 C8y C 22.3045 -11.2046
18 C8x C 22.3045 -12.6046
19 C8x C 23.5170 -13.3046
20 O2a O 21.1094 -10.5143
21 C1a C 19.9260 -11.1974
22 O7a O 24.0425 -15.2100
23 C7a C 23.6044 -16.5300
24 C1a C 22.2214 -16.8149
25 O6a O 24.5233 -17.5636
26 C1b C 29.7498 -17.3149
27 N1c N 30.4175 -18.5206
28 C1a C 31.8500 -18.5456
29 C1a C 29.6920 -19.7271
30 X Cl 24.6400 -19.5300
BOND 31
1 3 4 1
2 1 5 1
3 4 6 1
4 2 3 1
5 5 7 1
6 1 2 1
7 6 7 1
8 7 8 2
9 8 9 1
10 9 10 2
11 10 11 1
12 6 11 2
13 1 12 2
14 5 13 1
15 14 15 2
16 15 16 1
17 16 17 2
18 17 18 1
19 18 19 2
20 14 19 1
21 3 14 1 #Down
22 17 20 1
23 20 21 1
24 2 22 1 #Down
25 22 23 1
26 23 24 1
27 23 25 2
28 13 26 1
29 26 27 1
30 27 28 1
31 27 29 1
|
|---|
|
» Japanese version » Back
|