 | | DRUG: レベチラセタム | |
| エントリ |
|
|---|
| 一般名 |
レベチラセタム (JAN);
Levetiracetam (JAN/USP/INN) |
|---|
| 商品名 |
|
|---|
| 後発品 |
レベチラセタム (Meiji Seikaファルマ), レベチラセタム (Meiji Seikaファルマ), レベチラセタム (Meiji Seikaファルマ), レベチラセタム (キョーリンリメディオ), レベチラセタム (キョーリンリメディオ), レベチラセタム (サンド), レベチラセタム (ダイト), レベチラセタム (フェルゼンファーマ), レベチラセタム (共和薬品工業), レベチラセタム (共和薬品工業), レベチラセタム (共和薬品工業), レベチラセタム (日医工), レベチラセタム (日医工), レベチラセタム (日新製薬-山形), レベチラセタム (日新製薬-山形), レベチラセタム (日新製薬-山形), レベチラセタム (日本ジェネリック), レベチラセタム (日本ジェネリック), レベチラセタム (東和薬品), レベチラセタム (東和薬品), レベチラセタム (東和薬品), レベチラセタム (東和薬品), レベチラセタム (東和薬品), レベチラセタム (東和薬品), レベチラセタム (沢井製薬), レベチラセタム (沢井製薬), レベチラセタム (沢井製薬), レベチラセタム (陽進堂), レベチラセタム (高田製薬), レベチラセタム (高田製薬) |
|---|
| 米国の商品 |
|
|---|
| 後発品 |
LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (A-S Medication Solutions), LEVETIRACETAM (ACI Healthcare USA), LEVETIRACETAM (APNAR PHARMA LP), LEVETIRACETAM (Actavis Pharma), LEVETIRACETAM (Advanced Rx of Tennessee), LEVETIRACETAM (Advanced Rx of Tennessee), LEVETIRACETAM (American Health Packaging), LEVETIRACETAM (American Health Packaging), LEVETIRACETAM (American Health Packaging), LEVETIRACETAM (Amneal Pharmaceuticals LLC), LEVETIRACETAM (Amneal Pharmaceuticals LLC), LEVETIRACETAM (Apotex Corp.), LEVETIRACETAM (Armas Pharmaceuticals), LEVETIRACETAM (Ascend Laboratories), LEVETIRACETAM (Athenex Pharmaceutical Division), LEVETIRACETAM (Atlantic Biologicals Corp.), LEVETIRACETAM (Aurobindo Pharma Limited), LEVETIRACETAM (Aurobindo Pharma Limited), LEVETIRACETAM (B. Braun Medical), LEVETIRACETAM (Baxter Healthcare Corporation), LEVETIRACETAM (Bionpharma), LEVETIRACETAM (BluePoint Laboratories), LEVETIRACETAM (BluePoint Laboratories), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Bryant Ranch Prepack), LEVETIRACETAM (Camber Pharmaceuticals), LEVETIRACETAM (Camber Pharmaceuticals), LEVETIRACETAM (Caplin Steriles Limited), LEVETIRACETAM (Cardinal Health 107), LEVETIRACETAM (Cardinal Health 107), LEVETIRACETAM (Cardinal Health 107), LEVETIRACETAM (Cardinal Health 107), LEVETIRACETAM (Civica), LEVETIRACETAM (Coupler LLC), LEVETIRACETAM (Coupler LLC), LEVETIRACETAM (Coupler LLC), LEVETIRACETAM (Cranbury Pharmaceuticals), LEVETIRACETAM (DIRECT RX), LEVETIRACETAM (Dr. Reddy's Laboratories Limited), LEVETIRACETAM (Dr. Reddy's Laboratories Limited), LEVETIRACETAM (Dr.Reddy's Laboratories), LEVETIRACETAM (Dr.Reddy's Laboratories), LEVETIRACETAM (Eugia US LLC), LEVETIRACETAM (Eugia US LLC), LEVETIRACETAM (Exelan Pharmaceuticals), LEVETIRACETAM (Fresenius Kabi USA), LEVETIRACETAM (Fresenius Kabi USA), LEVETIRACETAM (Fresenius Kabi USA), LEVETIRACETAM (Gland Pharma Limited), LEVETIRACETAM (Golden State Medical Supply), LEVETIRACETAM (Granules Pharmaceuticals), LEVETIRACETAM (Guardian Drug Company), LEVETIRACETAM (Hikma Pharmaceuticals USA), LEVETIRACETAM (Hikma Pharmaceuticals USA), LEVETIRACETAM (Hisun Pharmaceuticals USA), LEVETIRACETAM (Hospira), LEVETIRACETAM (Hospira), LEVETIRACETAM (Hp Halden Pharma AS), LEVETIRACETAM (Hp Halden Pharma AS), LEVETIRACETAM (Hp Halden Pharma AS), LEVETIRACETAM (Kesin Pharma Corporation), LEVETIRACETAM (Lupin Pharmaceuticals), LEVETIRACETAM (Lupin Pharmaceuticals), LEVETIRACETAM (MAJOR PHARMACEUTICALS), LEVETIRACETAM (Major Pharmaceuticals), LEVETIRACETAM (Major Pharmaceuticals), LEVETIRACETAM (McKesson Corporation dba SKY Packaging), LEVETIRACETAM (Micro Labs Limited), LEVETIRACETAM (Mylan Institutional LLC), LEVETIRACETAM (Mylan Institutional), LEVETIRACETAM (Mylan Pharmaceuticals), LEVETIRACETAM (NCS HealthCare of KY), LEVETIRACETAM (Natco Pharma USA LLC), LEVETIRACETAM (NorthStar Rx LLC), LEVETIRACETAM (NorthStar RxLLC), LEVETIRACETAM (Northwind Pharmaceuticals), LEVETIRACETAM (Novadoz Pharmaceuticals LLC), LEVETIRACETAM (Novadoz Pharmaceuticals LLC), LEVETIRACETAM (Novadoz Pharmaceuticals LLC), LEVETIRACETAM (OWP Pharmaceuticals), LEVETIRACETAM (Omnivium Pharmaceuticals LLC), LEVETIRACETAM (PAI Holdings), LEVETIRACETAM (PD-Rx Pharmaceuticals), LEVETIRACETAM (PD-Rx Pharmaceuticals), LEVETIRACETAM (PD-Rx Pharmaceuticals), LEVETIRACETAM (Patrin Pharma), LEVETIRACETAM (Prasco Laboratories), LEVETIRACETAM (Precision Dose), LEVETIRACETAM (Preferred Pharmaceuticals), LEVETIRACETAM (Preferred Pharmaceuticals), LEVETIRACETAM (Preferred Pharmaceuticals), LEVETIRACETAM (Prinston Pharmaceutical), LEVETIRACETAM (Proficient Rx LP), LEVETIRACETAM (Proficient Rx LP), LEVETIRACETAM (Proficient Rx LP), LEVETIRACETAM (QUAGEN PHARMACEUTICALS LLC), LEVETIRACETAM (Quallent Pharmaceuticals Health LLC), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (REMEDYREPACK), LEVETIRACETAM (Redpharm Drug), LEVETIRACETAM (Rising Pharma Holdings), LEVETIRACETAM (Rising Pharma Holdings), LEVETIRACETAM (SOHM), LEVETIRACETAM (Sagent Pharmaceuticals), LEVETIRACETAM (Sagent Pharmaceuticals), LEVETIRACETAM (ScieGen Pharmaceuticals), LEVETIRACETAM (ScieGen Pharmaceuticals), LEVETIRACETAM (Solco Healthcare US), LEVETIRACETAM (Solco Healthcare US), LEVETIRACETAM (Solco Healthcare US), LEVETIRACETAM (Solco healthcare U.S.), LEVETIRACETAM (TAGI Pharma), LEVETIRACETAM (Taro Pharmaceuticals U.S.A.), LEVETIRACETAM (Torrent Pharmaceuticals Limited), LEVETIRACETAM (Westminster Pharmaceuticals), LEVETIRACETAM (XLCare Pharmaceuticals), LEVETIRACETAM ER (Direct_Rx), LEVETIRACETAM IN SODIUM CHLORIDE (Hikma Pharmaceuticals USA), LEVETIRACETAM IN SODIUM CHLORIDE (Nexus Pharmaceuticals LLC), LEVETIRACETAM SOLUTION (Chartwell RX) |
|---|
| 組成式 |
C8H14N2O2
|
|---|
| 質量 |
170.1055
|
|---|
| 分子量 |
170.21
|
|---|
| 構造式 |
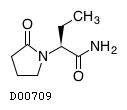
|
|---|
| Simcomp |
|
|---|
| クラス |
|
|---|
| コード |
|
|---|
| 効能 |
抗てんかん薬 |
|---|
| コメント |
ピラセタム誘導体
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 相互作用 |
|
|---|
| 構造マップ |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
1 神経系及び感覚器官用医薬品
11 中枢神経系用薬
113 抗てんかん剤
1139 その他の抗てんかん剤
D00709 レベチラセタム (JAN)
医療用医薬品のATC分類 [BR:jp08303]
N 神経系
N03 抗てんかん薬
N03A 抗てんかん薬
N03AX その他の抗てんかん薬
N03AX14 レベチラセタム
D00709 レベチラセタム (JAN) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
抗けいれん薬
抗けいれん薬, その他
レベチラセタム
D00709 レベチラセタム (JAN)
医薬品グループ [BR:jp08330]
精神神経系用薬
DG03199 抗てんかん薬
DG02038 ピラセタム系抗てんかん薬
D00709 レベチラセタム
医薬品クラス [BR:jp08332]
精神神経系用薬
DG03199 抗てんかん薬 [商品一覧]
D00709 レベチラセタム
ターゲットに基づく医薬品分類 [BR:jp08310]
トランスポーター
MFS スーパーファミリー
VNT ファミリー
SV2A
D00709 レベチラセタム (JAN) <JP/US>
日本の新薬 [jp08318.html]
新有効成分含有医薬品
D00709
日本・米国・欧州の新薬 [jp08328.html]
日米欧での承認状況
D00709
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|