| Entry |
|
|---|
| Name |
Doxorubicin hydrochloride (JP18/USP);
Adriacin (TN);
Adriamycin (TN);
Doxil (TN);
Rubex (TN) |
|---|
| Product |
|
|---|
| Generic |
ADRIAMYCIN (Hikma Pharmaceuticals USA), ADRIAMYCIN (Hikma Pharmaceuticals USA), ADRIAMYCIN (Hikma Pharmaceuticals USA), DOXORUBICIN HYDROCHLORIDE (Actavis Pharma), DOXORUBICIN HYDROCHLORIDE (Alembic Pharmaceuticals Limited), DOXORUBICIN HYDROCHLORIDE (Alembic Pharmaceuticals), DOXORUBICIN HYDROCHLORIDE (Amneal Pharmaceuticals LLC), DOXORUBICIN HYDROCHLORIDE (BluePoint Laboratories), DOXORUBICIN HYDROCHLORIDE (BluePoint Laboratories), DOXORUBICIN HYDROCHLORIDE (BluePoint Laboratories), DOXORUBICIN HYDROCHLORIDE (Dr. Reddy's Laboratories), DOXORUBICIN HYDROCHLORIDE (Dr. Reddy's Laboratories), DOXORUBICIN HYDROCHLORIDE (Fresenius Kabi USA), DOXORUBICIN HYDROCHLORIDE (Gland Pharma Limited), DOXORUBICIN HYDROCHLORIDE (Hikma Pharmaceuticals USA), DOXORUBICIN HYDROCHLORIDE (Hikma Pharmaceuticals USA), DOXORUBICIN HYDROCHLORIDE (Hikma Pharmaceuticals USA), DOXORUBICIN HYDROCHLORIDE (Lupin Pharmaceuticals), DOXORUBICIN HYDROCHLORIDE (Mylan Institutional LLC), DOXORUBICIN HYDROCHLORIDE (NorthStar RxLLC), DOXORUBICIN HYDROCHLORIDE (NorthStar RxLLC), DOXORUBICIN HYDROCHLORIDE (Sagent Pharmaceuticals), DOXORUBICIN HYDROCHLORIDE (Sun Pharmaceutical Industries), DOXORUBICIN HYDROCHLORIDE (Sun Pharmaceutical Industries), DOXORUBICIN HYDROCHLORIDE (Zydus Lifesciences Limited), DOXORUBICIN HYDROCHLORIDE (Zydus Pharmaceuticals USA), DOXORUBICIN HYDROCHLORIDE LIPOSOME (Baxter Healthcare Company) |
|---|
| Formula |
C27H29NO11. HCl
|
|---|
| Exact mass |
579.1507
|
|---|
| Mol weight |
579.98
|
|---|
| Structure |
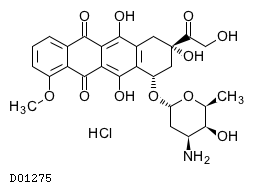
|
|---|
| Simcomp |
|
|---|
| Source |
Streptomyces peucetius [TAX: 1950]
|
|---|
| Class |
Antineoplastic
DG01682 Anthracycline antineoplastic
DG01529 Topoisomerase inhibitor
DG01527 Topoisomerase II inhibitor
|
|---|
| Remark |
| Therapeutic category: | 4235 |
| Product (DG00696): | D01275<JP/US> |
|
|---|
| Efficacy |
Antineoplastic, Topoisomerase II inhibitor |
|---|
| Disease |
Ovarian cancer [DS: H00027] AIDS-related Kaposi's sarcoma [DS: H00041] Multiple myeloma [DS: H00010] Acute lymphoblastic leukemia [DS: H00001 H00002] Acute myeloblastic leukemia [DS: H00003] Hodgkin lymphoma [DS: H00007] Non-Hodgkin lymphoma [DS: H02418] Breast cancer [DS: H00031] Wilms' tumor [DS: H02301] Neuroblastoma [DS: H00043] Soft tissue sarcoma [DS: H02427] Ovarian carcinoma [DS: H00027] Transitional cell bladder carcinoma [DS: H00022] Thyroid carcinoma [DS: H00032] Gastric carcinoma [DS: H00018] |
|---|
| Target |
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07042 | Antineoplastics - agents from natural products |
|
|---|
| Other map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
L ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
L01 ANTINEOPLASTIC AGENTS
L01D CYTOTOXIC ANTIBIOTICS AND RELATED SUBSTANCES
L01DB Anthracyclines and related substances
L01DB01 Doxorubicin
D01275 Doxorubicin hydrochloride (JP18/USP) <JP/US>
USP drug classification [BR:br08302]
Antineoplastics
Molecular Target Inhibitors
Topoisomerase Inhibitors
Doxorubicin
D01275 Doxorubicin hydrochloride (JP18/USP)
Therapeutic category of drugs in Japan [BR:br08301]
4 Agents affecting cellular function
42 Antineoplastics
423 Antibiotics
4235 Anthracycline antibiotics
D01275 Doxorubicin hydrochloride (JP18/USP)
Drug groups [BR:br08330]
Antineoplastic
DG01682 Anthracycline antineoplastic
DG00696 Doxorubicin
D01275 Doxorubicin hydrochloride
DG01529 Topoisomerase inhibitor
DG01527 Topoisomerase II inhibitor
DG00696 Doxorubicin
D01275 Doxorubicin hydrochloride
Target-based classification of drugs [BR:br08310]
Enzymes
Isomerases (EC5)
DNA topoisomerase
TOP2
D01275 Doxorubicin hydrochloride (JP18/USP) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D01275 Doxorubicin hydrochloride
D01275 Doxorubicin hydrochloride for injection
New drug approvals in Europe [br08329.html]
European public assessment reports (EPAR) authorised medicine
D01275
Drug groups [BR:br08330]
Antineoplastic
DG01682 Anthracycline antineoplastic
DG00696 Doxorubicin
DG01529 Topoisomerase inhibitor
DG01527 Topoisomerase II inhibitor
DG00696 Doxorubicin
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 40
1 C8x C 23.4525 -12.6014
2 C8x C 23.4525 -14.0018
3 C8y C 24.6429 -14.7020
4 C8y C 25.9033 -14.0018
5 C8y C 25.9033 -12.6014
6 C8x C 24.6429 -11.9012
7 C5x C 27.0936 -14.7020
8 C8y C 28.2840 -14.0018
9 C8y C 28.2840 -12.6014
10 C5x C 27.0936 -11.9012
11 C8y C 29.5443 -14.7020
12 C8y C 30.7347 -14.0018
13 C8y C 30.7347 -12.6014
14 C8y C 29.5443 -11.9012
15 C1y C 31.9250 -14.7020
16 C1x C 33.1854 -14.0018
17 C1z C 33.1854 -12.6014
18 C1x C 31.9250 -11.9012
19 O2a O 24.6429 -16.1024
20 C1a C 23.4525 -16.8026
21 O5x O 27.0936 -16.1024
22 O5x O 27.0936 -10.5008
23 O1a O 29.5443 -10.5008
24 O1a O 29.5443 -16.1024
25 O2a O 31.9250 -16.1024
26 C1y C 33.1154 -16.8026
27 C5a C 34.3758 -11.9012
28 C1b C 35.5661 -12.6014
29 O1a O 36.7565 -11.9012
30 O5a O 34.3758 -10.5008
31 O1a O 34.3758 -13.3016
32 C1x C 33.1154 -18.2030
33 C1y C 34.3758 -18.9032
34 C1y C 35.5661 -18.2030
35 C1y C 35.5661 -16.8026
36 O2x O 34.3758 -16.1024
37 N1a N 34.3930 -20.3035
38 C1a C 36.7818 -16.1073
39 O1a O 36.7818 -18.8983
40 X Cl 28.4940 -18.6932
BOND 43
1 1 2 2
2 2 3 1
3 3 4 2
4 4 5 1
5 5 6 2
6 1 6 1
7 4 7 1
8 7 8 1
9 8 9 1
10 9 10 1
11 5 10 1
12 8 11 2
13 11 12 1
14 12 13 2
15 13 14 1
16 9 14 2
17 12 15 1
18 15 16 1
19 16 17 1
20 17 18 1
21 13 18 1
22 3 19 1
23 19 20 1
24 7 21 2
25 10 22 2
26 14 23 1
27 11 24 1
28 26 25 1 #Down
29 15 25 1 #Down
30 17 27 1 #Up
31 27 28 1
32 28 29 1
33 27 30 2
34 17 31 1 #Down
35 26 32 1
36 32 33 1
37 33 34 1
38 34 35 1
39 35 36 1
40 26 36 1
41 33 37 1 #Up
42 35 38 1 #Up
43 34 39 1 #Up
|
|---|