 | | DRUG: Escitalopram oxalate | |
| Entry |
|
|---|
| Name |
Escitalopram oxalate (JAN/USP);
Lexapro (TN) |
|---|
| Product |
|
|---|
| Generic |
ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (A-S Medication Solutions), ESCITALOPRAM (Accord Healthcare), ESCITALOPRAM (Advanced Rx Pharmacy of Tennessee), ESCITALOPRAM (Advanced Rx Pharmacy of Tennessee), ESCITALOPRAM (American Health Packaging), ESCITALOPRAM (American Health Packaging), ESCITALOPRAM (Aphena Pharma Solutions - Tennessee), ESCITALOPRAM (Asclemed USA), ESCITALOPRAM (Aurobindo Pharma Limited), ESCITALOPRAM (BluePoint Laboratories), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Bryant Ranch Prepack), ESCITALOPRAM (Cardinal Health 107), ESCITALOPRAM (Coupler LLC), ESCITALOPRAM (Coupler LLC), ESCITALOPRAM (Coupler LLC), ESCITALOPRAM (Coupler LLC), ESCITALOPRAM (Coupler LLC), ESCITALOPRAM (Coupler LLC), ESCITALOPRAM (Direct_Rx), ESCITALOPRAM (Direct_Rx), ESCITALOPRAM (Direct_Rx), ESCITALOPRAM (Direct_Rx), ESCITALOPRAM (Jubilant Cadista Pharmacuticals), ESCITALOPRAM (Lupin Pharmaceuticals), ESCITALOPRAM (Macleods Pharmaceuticals Limited), ESCITALOPRAM (Major Pharmaceuticals), ESCITALOPRAM (NCS HealthCare of KY), ESCITALOPRAM (NCS HealthCare of KY), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (Northwind Pharmaceuticals), ESCITALOPRAM (NuCare Pharmaceuticals), ESCITALOPRAM (NuCare Pharmaceuticals), ESCITALOPRAM (NuCare Pharmaceuticals), ESCITALOPRAM (PD-Rx Pharmaceuticals), ESCITALOPRAM (PD-Rx Pharmaceuticals), ESCITALOPRAM (PD-Rx Pharmaceuticals), ESCITALOPRAM (Preferred Pharmaceuticals), ESCITALOPRAM (Preferred Pharmaceuticals), ESCITALOPRAM (Preferred Pharmaceuticals), ESCITALOPRAM (Preferred Pharmaceuticals), ESCITALOPRAM (Proficient Rx LP), ESCITALOPRAM (Proficient Rx LP), ESCITALOPRAM (Proficient Rx LP), ESCITALOPRAM (Proficient Rx LP), ESCITALOPRAM (Proficient Rx LP), ESCITALOPRAM (QPharma), ESCITALOPRAM (QPharma), ESCITALOPRAM (QPharma), ESCITALOPRAM (Quality Care Products), ESCITALOPRAM (Quality Care Products), ESCITALOPRAM (Quality Care Products), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (REMEDYREPACK), ESCITALOPRAM (RedPharm Drug), ESCITALOPRAM (RedPharm Drug), ESCITALOPRAM (RedPharm Drug), ESCITALOPRAM (RedPharm Drug), ESCITALOPRAM (Rising Pharma Holdings), ESCITALOPRAM (SOLCO HEALTHCARE US), ESCITALOPRAM (ST. MARY'S MEDICAL PARK PHARMACY), ESCITALOPRAM (ST. MARY'S MEDICAL PARK PHARMACY), ESCITALOPRAM (ST. MARY'S MEDICAL PARK PHARMACY), ESCITALOPRAM (Sun Pharmaceutical Industries), ESCITALOPRAM (Torrent Pharmaceuticals Limited), ESCITALOPRAM ORAL SOLUTION (Chartwell RX), ESCITALOPRAM OXALATE (A-S Medication Solutions), ESCITALOPRAM OXALATE (A-S Medication Solutions), ESCITALOPRAM OXALATE (Amneal Pharmaceuticals LLC), ESCITALOPRAM OXALATE (Amneal Pharmaceuticals LLC), ESCITALOPRAM OXALATE (Amneal Pharmaceuticals of New York LLC), ESCITALOPRAM OXALATE (Aphena Pharma Solutions - Tennessee), ESCITALOPRAM OXALATE (Ascent Pharmaceuticals), ESCITALOPRAM OXALATE (Aurobindo Pharma Limited), ESCITALOPRAM OXALATE (Bryant Ranch Prepack), ESCITALOPRAM OXALATE (Bryant Ranch Prepack), ESCITALOPRAM OXALATE (Bryant Ranch Prepack), ESCITALOPRAM OXALATE (Bryant Ranch Prepack), ESCITALOPRAM OXALATE (Bryant Ranch Prepack), ESCITALOPRAM OXALATE (Camber Pharmaceuticals), ESCITALOPRAM OXALATE (Cipla USA), ESCITALOPRAM OXALATE (Exelan Pharmaceuticals), ESCITALOPRAM OXALATE (Legacy Pharmaceutical Packaging), ESCITALOPRAM OXALATE (Macleods Pharmaceuticals Limited), ESCITALOPRAM OXALATE (NorthStar RxLLC), ESCITALOPRAM OXALATE (PD-Rx Pharmaceuticals), ESCITALOPRAM OXALATE (Pharmasource Meds), ESCITALOPRAM OXALATE (Preferred Pharmaceuticals), ESCITALOPRAM OXALATE (Proficient Rx LP), ESCITALOPRAM OXALATE (Proficient Rx LP), ESCITALOPRAM OXALATE (Quallent Pharmaceuticals Health LLC), ESCITALOPRAM OXALATE (REMEDYREPACK), ESCITALOPRAM OXALATE (Rising Pharma Holdings) |
|---|
| Formula |
C20H21FN2O. C2H2O4
|
|---|
| Exact mass |
414.1591
|
|---|
| Mol weight |
414.43
|
|---|
| Structure |
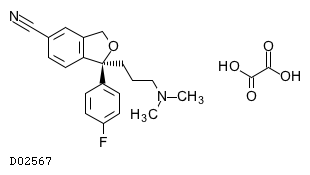
|
|---|
| Simcomp |
|
|---|
| Class |
Neuropsychiatric agent
DG01659 Selective serotonin reuptake inhibitor (SSRI)
Metabolizing enzyme substrate
DG01639 CYP2C19 substrate
DG01644 CYP2D6 substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG02953 AOX1 substrate
DG02917 MAO substrate
Metabolizing enzyme inhibitor
DG01645 CYP2D6 inhibitor
|
|---|
| Remark |
| Therapeutic category: | 1179 |
| Product (DG00947): | D02567<JP/US> |
|
|---|
| Efficacy |
Antidepressant, Selective serotonin reuptake inhibitor (SSRI) |
|---|
| Disease |
Major depressive disorder [DS: H01646] Generalized anxiety disorder [DS: H01662] |
|---|
| Comment |
Citalopram derivative
|
|---|
| Target |
|
|---|
| Pathway |
|
|---|
| Metabolism |
|
|---|
| Interaction |
CYP inhibition: CYP2D6 [HSA: 1565]
|
|---|
| Structure map |
| map07234 | Neurotransmitter transporter inhibitors |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
N NERVOUS SYSTEM
N06 PSYCHOANALEPTICS
N06A ANTIDEPRESSANTS
N06AB Selective serotonin reuptake inhibitors
N06AB10 Escitalopram
D02567 Escitalopram oxalate (JAN/USP) <JP/US>
USP drug classification [BR:br08302]
Antidepressants
SSRIs/SNRIs (Selective Serotonin Reuptake Inhibitors/Serotonin and Norepinephrine Reuptake Inhibitors)
Escitalopram
D02567 Escitalopram oxalate (JAN/USP)
Anxiolytics
SSRIs/SNRIs (Selective Serotonin Reuptake Inhibitors/Serotonin and Norepinephrine Reuptake Inhibitors)
Escitalopram
D02567 Escitalopram oxalate (JAN/USP)
Therapeutic category of drugs in Japan [BR:br08301]
1 Agents affecting nervous system and sensory organs
11 Agents affecting central nervous system
117 Psychotropics
1179 Others
D02567 Escitalopram oxalate (JAN/USP)
Drug groups [BR:br08330]
Neuropsychiatric agent
DG01659 Selective serotonin reuptake inhibitor (SSRI)
DG00947 Escitalopram
D02567 Escitalopram oxalate
Metabolizing enzyme substrate
DG01639 CYP2C19 substrate
DG00947 Escitalopram
D02567 Escitalopram oxalate
DG01644 CYP2D6 substrate
DG00947 Escitalopram
D02567 Escitalopram oxalate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00947 Escitalopram
D02567 Escitalopram oxalate
DG02953 AOX1 substrate
DG00947 Escitalopram
D02567 Escitalopram oxalate
DG02917 MAO substrate
DG00947 Escitalopram
D02567 Escitalopram oxalate
Metabolizing enzyme inhibitor
DG01645 CYP2D6 inhibitor
DG00947 Escitalopram
D02567 Escitalopram oxalate
Drug classes [BR:br08332]
Neuropsychiatric agent
DG01659 Selective serotonin reuptake inhibitor (SSRI)
D02567 Escitalopram oxalate
Target-based classification of drugs [BR:br08310]
Transporters
Solute carrier family
SLC6
SLC6A4 (HTT)
D02567 Escitalopram oxalate (JAN/USP) <JP/US>
New drug approvals in Japan [br08318.html]
Drugs with new active ingredients
D02567
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D02567
Pharmacogenomic biomarkers [br08341.html]
Polymorphisms and mutations affecting drug response
D02567
Drug groups [BR:br08330]
Neuropsychiatric agent
DG01659 Selective serotonin reuptake inhibitor (SSRI)
DG00947 Escitalopram
Metabolizing enzyme substrate
DG01639 CYP2C19 substrate
DG00947 Escitalopram
DG01644 CYP2D6 substrate
DG00947 Escitalopram
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00947 Escitalopram
DG02953 AOX1 substrate
DG00947 Escitalopram
DG02917 MAO substrate
DG00947 Escitalopram
Metabolizing enzyme inhibitor
DG01645 CYP2D6 inhibitor
DG00947 Escitalopram
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 30
1 C8x C 13.2300 -15.0500
2 C8y C 13.2300 -13.6500
3 C8x C 14.4200 -12.9500
4 C8y C 15.6800 -13.6500
5 C8y C 15.6800 -15.0500
6 C8x C 14.4200 -15.7500
7 C1x C 17.0100 -13.2300
8 O2x O 17.7800 -14.3500
9 C1z C 17.0100 -15.4700
10 C8y C 17.0100 -16.8700
11 C8x C 18.2000 -17.5700
12 C8x C 18.2000 -18.9700
13 C8y C 17.0800 -19.6700
14 C8x C 15.8200 -18.9700
15 C8x C 15.8200 -17.5700
16 X F 17.0800 -21.0700
17 C3b C 12.0400 -12.9500
18 N3a N 10.8500 -12.2500
19 C1b C 18.4100 -15.4700
20 C1b C 19.1100 -16.6824
21 C1b C 20.5100 -16.6600
22 N1c N 21.2293 -17.8611
23 C1a C 22.6100 -17.8388
24 C1a C 20.5491 -19.0842
25 O6a O 26.2500 -15.7500
26 C6a C 27.4624 -16.4500
27 C6a C 28.6579 -15.7596
28 O6a O 27.4625 -17.8498
29 O6a O 29.8453 -16.4451
30 O6a O 28.6580 -14.3503
BOND 31
1 1 2 1
2 2 3 2
3 3 4 1
4 4 5 2
5 5 6 1
6 1 6 2
7 4 7 1
8 7 8 1
9 8 9 1
10 5 9 1
11 9 10 1 #Up
12 10 11 2
13 11 12 1
14 12 13 2
15 13 14 1
16 14 15 2
17 10 15 1
18 13 16 1
19 2 17 1
20 17 18 3
21 9 19 1 #Down
22 19 20 1
23 20 21 1
24 21 22 1
25 22 23 1
26 22 24 1
27 25 26 1
28 26 27 1
29 26 28 2
30 27 29 1
31 27 30 2
|
|---|
|
» Japanese version » Back
|