 | | DRUG: クロベタゾールプロピオン酸エステル | |
| エントリ |
|
|---|
| 一般名 |
クロベタゾールプロピオン酸エステル (JP18);
プロピオン酸クロベタゾール;
Clobetasol propionate (JP18/USP) |
|---|
| 商品名 |
|
|---|
| 後発品 |
|
|---|
| 米国の商品 |
|
|---|
| 後発品 |
CLOBETASOL PROPIONATE (A-S Medication Solutions), CLOBETASOL PROPIONATE (A-S Medication Solutions), CLOBETASOL PROPIONATE (A-S Medication Solutions), CLOBETASOL PROPIONATE (A-S Medication Solutions), CLOBETASOL PROPIONATE (A-S Medication Solutions), CLOBETASOL PROPIONATE (A-S Medication Solutions), CLOBETASOL PROPIONATE (A-S Medication Solutions), CLOBETASOL PROPIONATE (Actavis Pharma), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals), CLOBETASOL PROPIONATE (Alembic Pharmaceuticals), CLOBETASOL PROPIONATE (Amneal Pharmaceuticals NY LLC), CLOBETASOL PROPIONATE (Amneal Pharmaceuticals NY LLC), CLOBETASOL PROPIONATE (Aurobindo Pharma Limited), CLOBETASOL PROPIONATE (AvKARE), CLOBETASOL PROPIONATE (AvKARE), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Bryant Ranch Prepack), CLOBETASOL PROPIONATE (Cosette Pharmaceuticals), CLOBETASOL PROPIONATE (DIRECT RX), CLOBETASOL PROPIONATE (E. Fougera & Co. a division of Fougera Pharmaceuticals), CLOBETASOL PROPIONATE (E. Fougera & Co. a division of Fougera Pharmaceuticals), CLOBETASOL PROPIONATE (EMOLLIENT) (E. Fougera & Co. a division of Fougera Pharmaceuticals), CLOBETASOL PROPIONATE (EMOLLIENT) (The J. Molner Company LLC), CLOBETASOL PROPIONATE (Encube Ethicals), CLOBETASOL PROPIONATE (Encube Ethicals), CLOBETASOL PROPIONATE (Glenmark Pharmaceutcials), CLOBETASOL PROPIONATE (Glenmark Pharmaceuticals), CLOBETASOL PROPIONATE (Glenmark Pharmaceuticals), CLOBETASOL PROPIONATE (Glenmark Pharmaceuticals), CLOBETASOL PROPIONATE (Glenmark Pharmaceuticals), CLOBETASOL PROPIONATE (Glenmark Pharmaceuticals), CLOBETASOL PROPIONATE (Golden State Medical Supply), CLOBETASOL PROPIONATE (INA Pharmaceutics), CLOBETASOL PROPIONATE (Lupin Pharmaceuticals), CLOBETASOL PROPIONATE (Lupin Pharmaceuticals), CLOBETASOL PROPIONATE (Lupin Pharmaceuticals), CLOBETASOL PROPIONATE (Lupin Pharmaceuticals), CLOBETASOL PROPIONATE (Macleods Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Macleods Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Macleods Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Mayne Pharma), CLOBETASOL PROPIONATE (Micro Labs Limited), CLOBETASOL PROPIONATE (NORTHSTAR RX LLC), CLOBETASOL PROPIONATE (Northstar Rx LLC), CLOBETASOL PROPIONATE (Novel Laboratories), CLOBETASOL PROPIONATE (Novel Laboratories), CLOBETASOL PROPIONATE (NuCare Pharmaceuticals), CLOBETASOL PROPIONATE (Padagis Israel Pharmaceuticals), CLOBETASOL PROPIONATE (Padagis Israel Pharmaceuticals), CLOBETASOL PROPIONATE (Padagis Israel Pharmaceuticals), CLOBETASOL PROPIONATE (Padagis Israel Pharmaceuticals), CLOBETASOL PROPIONATE (Preferred Pharmaceuticals), CLOBETASOL PROPIONATE (Proficient Rx LP), CLOBETASOL PROPIONATE (Quagen Pharmaceuticals LLC), CLOBETASOL PROPIONATE (Sun Pharmaceutical Industries), CLOBETASOL PROPIONATE (Sun Pharmaceutical Industries), CLOBETASOL PROPIONATE (Sun Pharmaceutical Industries), CLOBETASOL PROPIONATE (Sun Pharmaceutical Industries), CLOBETASOL PROPIONATE (Sun Pharmaceutical Industries), CLOBETASOL PROPIONATE (Sun Pharmaceutical Indutries), CLOBETASOL PROPIONATE (Taro Pharmaceuticals U.S.A.), CLOBETASOL PROPIONATE (Torrent Pharmaceuticals Limited), CLOBETASOL PROPIONATE (Viona Pharmaceuticals), CLOBETASOL PROPIONATE (Xiromed), CLOBETASOL PROPIONATE (Xiromed), CLOBETASOL PROPIONATE (Zydus Lifesciences Limited), CLOBETASOL PROPIONATE (Zydus Lifesciences Limited), CLOBETASOL PROPIONATE (Zydus Lifesciences Limited), CLOBETASOL PROPIONATE (Zydus Pharmaceuticals USA), CLOBETASOL PROPIONATE (Zydus Pharmaceuticals USA), CLOBETASOL PROPIONATE CREAM 0.05% (Preferred Pharmaceuticals), CLOBETASOL PROPIONATE CREAM USP, 0.05% (A-S Medication Solutions), CLOBETASOL PROPIONATE CREAM USP, 0.05% (A-S Medication Solutions), CLOBETASOL PROPIONATE CREAM USP, 0.05% (Bryant Ranch Prepack), CLOBETASOL PROPIONATE CREAM USP, 0.05% (Encube Ethicals), CLOBETASOL PROPIONATE CREAM USP, 0.05% (Northstar Rx LLC), CLOBETASOL PROPIONATE CREAM USP, 0.05% (REMEDYREPACK), CLOBETASOL PROPIONATE CREAM, 0.05% (Proficient Rx LP), CLODAN (Medimetriks Pharmaceuticals), TOVET (EMOLLIENT FORMULATION) (Medimetriks Pharmaceuticals) |
|---|
| 組成式 |
C25H32ClFO5
|
|---|
| 質量 |
466.1922
|
|---|
| 分子量 |
466.97
|
|---|
| 構造式 |
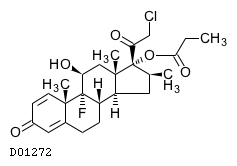
|
|---|
| Simcomp |
|
|---|
| クラス |
|
|---|
| コード |
| 商品 (DG00422): | D01272<JP/US> |
|
|---|
| 効能 |
抗炎症薬, グルココルチコイド受容体作動薬 |
|---|
| 疾患 |
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 相互作用 |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
2 個々の器官系用医薬品
26 外皮用薬
264 鎮痛、鎮痒、収斂、消炎剤
2646 副腎皮質ホルモン製剤
D01272 クロベタゾールプロピオン酸エステル (JP18); プロピオン酸クロベタゾール
医療用医薬品のATC分類 [BR:jp08303]
D 皮膚科用薬
D07 副腎皮質ステロイド、皮膚科用製剤
D07A 副腎皮質ステロイド、単剤
D07AD 副腎皮質ステロイド、最強 (グループ IV)
D07AD01 クロベタゾール
D01272 クロベタゾールプロピオン酸エステル (JP18) <JP/US>
S 感覚器
S01 眼科用薬
S01B 抗炎症薬
S01BA 副腎皮質ステロイド、単剤
S01BA17 クロベタゾール
D01272 クロベタゾールプロピオン酸エステル (JP18) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
皮膚科用薬
皮膚炎・そう痒治療薬
糖質コルチコイド, ベリーハイポテンシー
クロベタゾール
D01272 クロベタゾールプロピオン酸エステル (JP18)
医薬品グループ [BR:jp08330]
抗炎症薬
DG01955 副腎皮質ステロイド
DG02068 糖質コルチコイド
DG00422 クロベタゾール
D01272 クロベタゾールプロピオン酸エステル
皮膚科用薬
DG03243 アトピー性皮膚炎治療薬
D01272 クロベタゾールプロピオン酸エステル
医薬品クラス [BR:jp08332]
抗炎症薬
DG01955 副腎皮質ステロイド [商品一覧]
D01272 クロベタゾールプロピオン酸エステル
皮膚科用薬
DG03243 アトピー性皮膚炎治療薬 [商品一覧]
D01272 クロベタゾールプロピオン酸エステル
ターゲットに基づく医薬品分類 [BR:jp08310]
核内受容体
エストロゲン様受容体
3-ケトステロイド受容体
NR3C1 (GR)
D01272 クロベタゾールプロピオン酸エステル (JP18) <JP/US>
日本薬局方収載医薬品 [BR:jp08311]
化学薬品等
D01272 クロベタゾールプロピオン酸エステル
ステロイド外用薬の強さ [jp08317.html]
日本の分類
D01272
米国の分類
D01272
医薬品グループ [BR:jp08330]
抗炎症薬
DG01955 副腎皮質ステロイド
DG02068 糖質コルチコイド
DG00422 クロベタゾール
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|