 | | DRUG: アトルバスタチンカルシウム水和物 | |
| エントリ |
|
|---|
| 一般名 |
アトルバスタチンカルシウム水和物 (JP18);
アトルバスタチンカルシウム;
Atorvastatin calcium (USP);
Atorvastatin calcium hydrate (JP18);
Atorvastatin calcium trihydrate |
|---|
| 商品名 |
|
|---|
| 後発品 |
アトルバスタチン (Meファルマ), アトルバスタチン (Meファルマ), アトルバスタチン (Meファルマ), アトルバスタチン (Meファルマ), アトルバスタチン (キョーリンリメディオ), アトルバスタチン (サンド), アトルバスタチン (ニプロ), アトルバスタチン (ヴィアトリス・ヘルスケア), アトルバスタチン (全星薬品工業), アトルバスタチン (日医工), アトルバスタチン (日新製薬-山形), アトルバスタチン (日新製薬-山形), アトルバスタチン (日本ケミファ), アトルバスタチン (日本ケミファ), アトルバスタチン (日本ジェネリック), アトルバスタチン (東和薬品), アトルバスタチン (沢井製薬), アトルバスタチン (第一三共エスファ), アトルバスタチン (辰巳化学), アトルバスタチン (陽進堂), アトルバスタチン (鶴原製薬) |
|---|
| 米国の商品 |
|
|---|
| 後発品 |
ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (A-S Medication Solutions), ATORVASTATIN CALCIUM (Accord Healthcare), ATORVASTATIN CALCIUM (Aphena Pharma Solutions - Tennessee), ATORVASTATIN CALCIUM (Aphena Pharma Solutions - Tennessee), ATORVASTATIN CALCIUM (Aphena Pharma Solutions - Tennessee), ATORVASTATIN CALCIUM (Aphena Pharma Solutions - Tennessee), ATORVASTATIN CALCIUM (Aphena Pharma Solutions - Tennessee), ATORVASTATIN CALCIUM (Ascend Laboratories), ATORVASTATIN CALCIUM (Asclemed USA), ATORVASTATIN CALCIUM (Aurobindo Pharma Limited), ATORVASTATIN CALCIUM (Biocon Pharma Inc.,), ATORVASTATIN CALCIUM (Biocon Pharma), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Bryant Ranch Prepack), ATORVASTATIN CALCIUM (Cadila Pharmaceuticals Limited), ATORVASTATIN CALCIUM (Camber Pharmaceuticals), ATORVASTATIN CALCIUM (Cardinal Health 107), ATORVASTATIN CALCIUM (Cipla USA Inc.,), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (Coupler LLC), ATORVASTATIN CALCIUM (DIRECT RX), ATORVASTATIN CALCIUM (DirectRX), ATORVASTATIN CALCIUM (Direct_Rx), ATORVASTATIN CALCIUM (Direct_Rx), ATORVASTATIN CALCIUM (Direct_Rx), ATORVASTATIN CALCIUM (Direct_rx), ATORVASTATIN CALCIUM (Dr. Reddy's Laboratories Limited), ATORVASTATIN CALCIUM (Dr. Reddy's Laboratories Limited), ATORVASTATIN CALCIUM (Dr. Reddy's Laboratories), ATORVASTATIN CALCIUM (Dr. Reddy's Laboratories), ATORVASTATIN CALCIUM (Golden State Medical Supply), ATORVASTATIN CALCIUM (Laurus Labs Limited), ATORVASTATIN CALCIUM (Lepu Pharmaceutical Technology), ATORVASTATIN CALCIUM (Lifestar Pharma LLC), ATORVASTATIN CALCIUM (Lupin Pharmaceuticals), ATORVASTATIN CALCIUM (MSN LABORATORIES PRIVATE LIMITED), ATORVASTATIN CALCIUM (Micro Labs Limited), ATORVASTATIN CALCIUM (Mylan Institutional), ATORVASTATIN CALCIUM (Mylan Pharmaceuticals), ATORVASTATIN CALCIUM (NIVAGEN PHARMACEUTICALS), ATORVASTATIN CALCIUM (NorthStar RxLLC), ATORVASTATIN CALCIUM (Northwind Pharmaceuticals), ATORVASTATIN CALCIUM (Northwind Pharmaceuticals), ATORVASTATIN CALCIUM (Northwind Pharmaceuticals), ATORVASTATIN CALCIUM (Northwind Pharmaceuticals), ATORVASTATIN CALCIUM (Northwind Pharmaceuticals), ATORVASTATIN CALCIUM (Novadoz Pharmaceuticals LLC), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (NuCare Pharmaceuticals), ATORVASTATIN CALCIUM (PD-Rx Pharmaceuticals), ATORVASTATIN CALCIUM (PD-Rx Pharmaceuticals), ATORVASTATIN CALCIUM (PD-Rx Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Preferred Pharmaceuticals), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (Proficient Rx LP), ATORVASTATIN CALCIUM (QPharma), ATORVASTATIN CALCIUM (QPharma), ATORVASTATIN CALCIUM (QPharma), ATORVASTATIN CALCIUM (Quality Care Products), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (REMEDYREPACK), ATORVASTATIN CALCIUM (Radha Pharmaceuticals), ATORVASTATIN CALCIUM (RedPharm Drug), ATORVASTATIN CALCIUM (RedPharm Drug), ATORVASTATIN CALCIUM (RedPharm Drug), ATORVASTATIN CALCIUM (Redpharm Drug), ATORVASTATIN CALCIUM (Rising Pharma Holdings), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ST. MARY'S MEDICAL PARK PHARMACY), ATORVASTATIN CALCIUM (ScieGen Pharmaceuticals), ATORVASTATIN CALCIUM (St Mary's Medical Park Pharmacy), ATORVASTATIN CALCIUM (St. Mary's Medical Park Pharmacy), ATORVASTATIN CALCIUM (Sun Pharmaceutical Industries), ATORVASTATIN CALCIUM (Teva Pharmaceuticals USA), ATORVASTATIN CALCIUM (Zydus Lifesciences Limited), ATORVASTATIN CALCIUM (Zydus Lifesciences Limited), ATORVASTATIN CALCIUM (Zydus Pharmaceuticals USA), ATORVASTATIN CALCIUM (Zydus Pharmaceuticals USA) |
|---|
| 組成式 |
(C33H34FN2O5)2. 3H2O. Ca
|
|---|
| 質量 |
1208.4846
|
|---|
| 分子量 |
1209.38
|
|---|
| 構造式 |
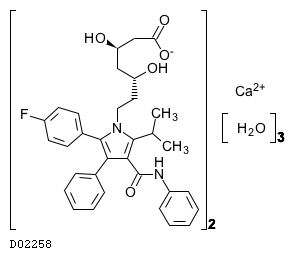
|
|---|
| Simcomp |
|
|---|
| クラス |
|
|---|
| コード |
| 商品 (DG00355): | D07474<US> D02258<JP/US> D11095<US> |
| 商品 (mixture): | D08488<JP/US> D10385<JP/US> |
|
|---|
| 効能 |
脂質異常症治療薬, HMG-CoA還元酵素阻害薬 |
|---|
| 疾患 |
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 代謝 |
Enzyme: CYP3A4 [HSA: 1576]
Transporter: ABCB1 [HSA: 5243], ABCG2 [HSA: 9429], SLCO1B1 [HSA: 10599], SLCO1B3 [HSA: 28234]
|
|---|
| 相互作用 |
|
|---|
| 構造マップ |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
2 個々の器官系用医薬品
21 循環器官用薬
218 高脂血症用剤
2189 その他の高脂血症用剤
D02258 アトルバスタチンカルシウム水和物 (JP18); アトルバスタチンカルシウム
医療用医薬品のATC分類 [BR:jp08303]
C 循環器系
C10 脂質修飾剤
C10A 脂質修飾剤、単剤
C10AA HMG-CoA 還元酵素阻害薬
C10AA05 アトルバスタチン
D02258 アトルバスタチンカルシウム水和物 (JP18) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
心血管治療薬
脂質異常症治療薬, ヒドロキシメチルグルタリルコエンザイムA還元酵素阻害薬
アトルバスタチン
D02258 アトルバスタチンカルシウム水和物 (JP18)
医薬品グループ [BR:jp08330]
脂質降下薬
DG01946 脂質低下薬
DG01660 HMG-CoA還元酵素阻害薬
DG00355 アトルバスタチン
D02258 アトルバスタチンカルシウム水和物
代謝酵素基質薬
DG01633 CYP3A/CYP3A4基質薬
DG02913 CYP3A4基質薬
DG00355 アトルバスタチン
D02258 アトルバスタチンカルシウム水和物
トランスポーター基質薬
DG01665 ABCB1基質薬
DG00355 アトルバスタチン
D02258 アトルバスタチンカルシウム水和物
DG01913 ABCG2基質薬
DG00355 アトルバスタチン
D02258 アトルバスタチンカルシウム水和物
DG02856 SLCO1B1基質薬
DG00355 アトルバスタチン
D02258 アトルバスタチンカルシウム水和物
DG02932 SLCO1B3基質薬
DG00355 アトルバスタチン
D02258 アトルバスタチンカルシウム水和物
医薬品クラス [BR:jp08332]
心血管系用薬
DG01946 脂質低下薬 [商品一覧]
D02258 アトルバスタチンカルシウム水和物
ターゲットに基づく医薬品分類 [BR:jp08310]
酵素
オキシドレダクターゼ (EC1)
デヒドロゲナーゼ
HMGCR
D02258 アトルバスタチンカルシウム水和物 (JP18) <JP/US>
日本薬局方収載医薬品 [BR:jp08311]
化学薬品等
D02258 アトルバスタチンカルシウム水和物
D02258 アトルバスタチンカルシウム錠
日本の新薬 [jp08318.html]
新有効成分含有医薬品
D02258
薬物代謝酵素とトランスポーター [jp08309.html]
薬物代謝酵素
D02258
薬物トランスポーター
D02258
医薬品グループ [BR:jp08330]
脂質降下薬
DG01946 脂質低下薬
DG01660 HMG-CoA還元酵素阻害薬
DG00355 アトルバスタチン
代謝酵素基質薬
DG01633 CYP3A/CYP3A4基質薬
DG02913 CYP3A4基質薬
DG00355 アトルバスタチン
トランスポーター基質薬
DG01665 ABCB1基質薬
DG00355 アトルバスタチン
DG01913 ABCG2基質薬
DG00355 アトルバスタチン
DG02856 SLCO1B1基質薬
DG00355 アトルバスタチン
DG02932 SLCO1B3基質薬
DG00355 アトルバスタチン
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|