 | | DRUG: Lidocaine hydrochloride | |
| Entry |
|
|---|
| Name |
Lidocaine hydrochloride (JAN/USP);
Dalcaine (TN);
Xylocaine (TN) |
|---|
| Product |
AKTEN (Thea Pharma), LIDOCAINE (Fresenius Kabi USA), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI AND DEXTROSE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE AND DEXTROSE (Baxter Healthcare Corporation), LIDOCAINE HYDROCHLORIDE AND DEXTROSE (HF Acquisition Co LLC), XYLOCAIN (LIDOCAINE HCL ) (HF Acquisition Co LLC), XYLOCAINE (ASCLEMED USA), XYLOCAINE (Asclemed USA), XYLOCAINE (Fresenius Kabi USA), XYLOCAINE (Fresenius Kabi USA), XYLOCAINE (General Injectables & Vaccines), XYLOCAINE (General Injectables and Vaccines), XYLOCAINE (HF Acquisition Co LLC), XYLOCAINE (HF Acquisition Co LLC), XYLOCAINE (Henry Schein), XYLOCAINE (Sportpharm), XYLOCAINE MPF (HF Acquisition Co LLC), XYLOCAINE MPF (HF Acquisition Co LLC), XYLOCAINE MPF (HF Acquisition Co LLC), XYLOCAINE- MPF (LIDOCAINE HCL ) (HF Acquisition Co LLC) |
|---|
| Generic |
0.5% LIDOCAINE HCL (HF Acquisition Co LLC), GLYDO (Sagent Pharmaceuticals), GLYDO- LIDOCAINE HYDROCHLORIDE JELLY (HF Acquisition Co. LLC), LIDOCAINE (A-S Medication Solutions), LIDOCAINE (ARMAS PHARMACEUTICALS), LIDOCAINE (Fresenius Kabi USA), LIDOCAINE (Hikma Pharmaceuticals USA), LIDOCAINE (Medical Purchasing Solutions), LIDOCAINE (Medical Purchasing Solutions), LIDOCAINE (Sportpharm), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCL (HF Acquisition Co LLC), LIDOCAINE HCL (HF Acquisition Co LLC), LIDOCAINE HCL (Nextsource Pharma), LIDOCAINE HCL (RXnSupplies Corporation), LIDOCAINE HYDROCHLORIDE (A-S Medication Solutions), LIDOCAINE HYDROCHLORIDE (ATLANTIC BIOLOGICALS CORP.), LIDOCAINE HYDROCHLORIDE (ATLANTIC BIOLOGICALS CORP.), LIDOCAINE HYDROCHLORIDE (Advagen Pharma), LIDOCAINE HYDROCHLORIDE (Advanced Rx Pharmacy of Tennessee), LIDOCAINE HYDROCHLORIDE (American Health Packaging), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Atlantic Biologicals Corp.), LIDOCAINE HYDROCHLORIDE (B. Braun Medical), LIDOCAINE HYDROCHLORIDE (Brookfield Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (Bryant Ranch Prepack), LIDOCAINE HYDROCHLORIDE (Bryant Ranch Prepack), LIDOCAINE HYDROCHLORIDE (Camber Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (Camber Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (Cardinal Health 107), LIDOCAINE HYDROCHLORIDE (Cardinal Health 107), LIDOCAINE HYDROCHLORIDE (Chartwell RX), LIDOCAINE HYDROCHLORIDE (Civica), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Hikma Pharmaceuticals USA), LIDOCAINE HYDROCHLORIDE (Hikma Pharmaceuticals USA), LIDOCAINE HYDROCHLORIDE (Hospira), LIDOCAINE HYDROCHLORIDE (Huons), LIDOCAINE HYDROCHLORIDE (International Medication Systems), LIDOCAINE HYDROCHLORIDE (International Medication Systems), LIDOCAINE HYDROCHLORIDE (International Medication Systems), LIDOCAINE HYDROCHLORIDE (Lannett Company), LIDOCAINE HYDROCHLORIDE (Lifestar Pharma LLC), LIDOCAINE HYDROCHLORIDE (Lifestar Pharma LLC), LIDOCAINE HYDROCHLORIDE (Marlex Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (McKesson Corporation dba SKY Packaging), LIDOCAINE HYDROCHLORIDE (McKesson Corporation dba SKY Packaging), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (NorthStar RxLLC), LIDOCAINE HYDROCHLORIDE (NorthStar RxLLC), LIDOCAINE HYDROCHLORIDE (Novagenix Labs LLC), LIDOCAINE HYDROCHLORIDE (Novitium Pharma LLC), LIDOCAINE HYDROCHLORIDE (NuCare Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (NuCare Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (NuCare Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (PAI Holdings), LIDOCAINE HYDROCHLORIDE (PAI Holdings), LIDOCAINE HYDROCHLORIDE (Precision Dose), LIDOCAINE HYDROCHLORIDE (Preferred Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (ProPharma Distribution), LIDOCAINE HYDROCHLORIDE (ProPharma Distribution), LIDOCAINE HYDROCHLORIDE (REMEDYREPACK), LIDOCAINE HYDROCHLORIDE (Spectra Medical Devices), LIDOCAINE HYDROCHLORIDE (Sportpharm), LIDOCAINE HYDROCHLORIDE (The J. Molner Company LLC), LIDOCAINE HYDROCHLORIDE (Wockhardt USA LLC.), LIDOCAINE VISCOUS (A-S Medication Solutions), LIDOCAINE VISCOUS (Hikma Pharmaceuticals USA), NA (SINTETICA SA), NA (Sintetica US LLC) |
|---|
| Formula |
C14H22N2O. HCl
|
|---|
| Exact mass |
270.1499
|
|---|
| Mol weight |
270.80
|
|---|
| Structure |
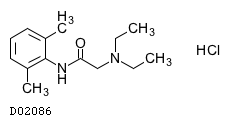
|
|---|
| Simcomp |
|
|---|
| Class |
Cardiovascular agent
DG01653 Antiarrhythmics
DG01652 Class I antiarrhythmic agent
DG01651 Class Ib antiarrhythmic agent
Neuropsychiatric agent
DG02030 Anesthetics
DG01675 Local anesthetic
DG01673 Amide type local anesthetic
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
|
|---|
| Remark |
| Product (DG00196): | D00358<JP/US> D02086<JP/US> D08127<JP/US> |
| Product (mixture): | D04052<JP/US> D04813<JP/US> |
|
|---|
| Efficacy |
Anesthetic (local), Antiarrhythmic, Sodium channel blocker |
|---|
| Comment |
Anilide derivative
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04261 | Adrenergic signaling in cardiomyocytes |
|
|---|
| Metabolism |
Enzyme: CYP1A2 [HSA: 1544], CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
|
|---|
| Structure map |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
C CARDIOVASCULAR SYSTEM
C01 CARDIAC THERAPY
C01B ANTIARRHYTHMICS, CLASS I AND III
C01BB Antiarrhythmics, class Ib
C01BB01 Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
C05 VASOPROTECTIVES
C05A AGENTS FOR TREATMENT OF HEMORRHOIDS AND ANAL FISSURES FOR TOPICAL USE
C05AD Local anesthetics
C05AD01 Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
D DERMATOLOGICALS
D04 ANTIPRURITICS, INCL. ANTIHISTAMINES, ANESTHETICS, ETC.
D04A ANTIPRURITICS, INCL. ANTIHISTAMINES, ANESTHETICS, ETC.
D04AB Anesthetics for topical use
D04AB01 Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
N NERVOUS SYSTEM
N01 ANESTHETICS
N01B ANESTHETICS, LOCAL
N01BB Amides
N01BB02 Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
R RESPIRATORY SYSTEM
R02 THROAT PREPARATIONS
R02A THROAT PREPARATIONS
R02AD Anesthetics, local
R02AD02 Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
S SENSORY ORGANS
S01 OPHTHALMOLOGICALS
S01H LOCAL ANESTHETICS
S01HA Local anesthetics
S01HA07 Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
S02 OTOLOGICALS
S02D OTHER OTOLOGICALS
S02DA Analgesics and anesthetics
S02DA01 Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
USP drug classification [BR:br08302]
Anesthetics
Local Anesthetics
Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP)
Therapeutic category of drugs in Japan [BR:br08301]
1 Agents affecting nervous system and sensory organs
12 Agents affecting peripheral nervous system
121 Local anesthetics
1214 Xylidines
D02086 Lidocaine hydrochloride (JAN/USP)
13 Agents affecting sensory organs
131 Ophthalmic agents
1313 Ophthalmic local anesthetics
D02086 Lidocaine hydrochloride (JAN/USP)
2 Agents affecting individual organs
21 Cardiovascular agents
212 Antiarrhythmic agents
2129 Others
D02086 Lidocaine hydrochloride (JAN/USP)
Classification of Japanese OTC drugs [BR:br08313]
Agents for urogenital organs and anus
30 Hemorrhoid drugs for external use
D02086 Lidocaine hydrochloride (JAN/USP)
Agents for integumentary system
57 Analgesic, antipruritic, astringent, and anti-inflammatory remedies (incl. gel patches)
D02086 Lidocaine hydrochloride (JAN/USP)
Risk category of Japanese OTC drugs [BR:br08312]
Second-class OTC drugs
Inorganic and organic chemicals
Lidocaine
D02086 Lidocaine hydrochloride (JAN/USP)
Drug groups [BR:br08330]
Cardiovascular agent
DG01653 Antiarrhythmics
DG01652 Class I antiarrhythmic agent
DG01651 Class Ib antiarrhythmic agent
DG00196 Lidocaine
D02086 Lidocaine hydrochloride
Neuropsychiatric agent
DG02030 Anesthetics
DG01675 Local anesthetic
DG01673 Amide type local anesthetic
DG00196 Lidocaine
D02086 Lidocaine hydrochloride
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00196 Lidocaine
D02086 Lidocaine hydrochloride
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00196 Lidocaine
D02086 Lidocaine hydrochloride
Drug classes [BR:br08332]
Cardiovascular agent
DG01653 Antiarrhythmics
D02086 Lidocaine hydrochloride
Target-based classification of drugs [BR:br08310]
Ion channels
Voltage-gated ion channels
Sodium channels
SCN1A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN2A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN3A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN4A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN5A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN8A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN9A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN10A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
SCN11A
D02086 Lidocaine hydrochloride (JAN/USP) <JP/US>
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D02086
Drug groups [BR:br08330]
Cardiovascular agent
DG01653 Antiarrhythmics
DG01652 Class I antiarrhythmic agent
DG01651 Class Ib antiarrhythmic agent
DG00196 Lidocaine
Neuropsychiatric agent
DG02030 Anesthetics
DG01675 Local anesthetic
DG01673 Amide type local anesthetic
DG00196 Lidocaine
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00196 Lidocaine
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00196 Lidocaine
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 18
1 C8y C 27.4132 -17.1509
2 N1b N 28.6465 -17.8641
3 C8y C 26.1855 -17.8699
4 C8y C 27.4132 -15.7304
5 C5a C 29.8742 -17.1450
6 C8x C 24.9579 -17.1509
7 C1a C 26.1914 -19.2846
8 C8x C 26.1855 -15.0230
9 C1a C 28.6348 -15.0230
10 C1b C 31.1017 -17.8641
11 O5a O 29.9091 -16.0694
12 C8x C 24.9579 -15.7304
13 N1c N 32.3352 -17.1450
14 C1b C 33.5686 -17.8641
15 C1b C 32.3352 -15.7304
16 C1a C 34.7845 -17.1450
17 C1a C 33.5686 -15.0172
18 X Cl 39.1297 -16.5898
BOND 17
1 1 2 1
2 1 3 2
3 1 4 1
4 2 5 1
5 3 6 1
6 3 7 1
7 4 8 2
8 4 9 1
9 5 10 1
10 5 11 2
11 6 12 2
12 10 13 1
13 13 14 1
14 13 15 1
15 14 16 1
16 15 17 1
17 8 12 1
|
|---|
|
» Japanese version » Back
|