 | | DRUG: 塩酸デュロキセチン | |
| エントリ |
|
|---|
| 一般名 |
塩酸デュロキセチン (JAN);
デュロキセチン塩酸塩;
Duloxetine hydrochloride (JAN/USP) |
|---|
| 商品名 |
|
|---|
| 後発品 |
デュロキセチン (Meiji Seikaファルマ), デュロキセチン (キョーリンリメディオ), デュロキセチン (ダイト), デュロキセチン (ニプロ), デュロキセチン (ニプロ), デュロキセチン (三笠製薬), デュロキセチン (共創未来ファーマ), デュロキセチン (共和薬品工業), デュロキセチン (大原薬品工業), デュロキセチン (大原薬品工業), デュロキセチン (富士化学工業), デュロキセチン (日医工岐阜工場), デュロキセチン (日新製薬-山形), デュロキセチン (東和薬品), デュロキセチン (東和薬品), デュロキセチン (沢井製薬), デュロキセチン (第一三共エスファ), デュロキセチン (長生堂製薬), デュロキセチン (陽進堂), デュロキセチン (高田製薬) |
|---|
| 米国の商品 |
|
|---|
| 後発品 |
DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (A-S Medication Solutions), DULOXETINE (Actavis Pharma), DULOXETINE (Advanced Rx Pharmacy of Tennessee), DULOXETINE (Advanced Rx Pharmacy of Tennessee), DULOXETINE (Advanced Rx of Tennessee), DULOXETINE (Ajanta Pharma USA), DULOXETINE (American Health Packaging), DULOXETINE (Aphena Pharma Solutions - Tennessee), DULOXETINE (Aphena Pharma Solutions - Tennessee), DULOXETINE (Aphena Pharma Solutions - Tennessee), DULOXETINE (Aphena Pharma Solutions - Tennessee), DULOXETINE (Aphena Pharma Solutions - Tennessee), DULOXETINE (Aphena Pharma Solutions - Tennessee), DULOXETINE (Aphena Pharma Solutions - Tennessee), DULOXETINE (Ascend Laboratories), DULOXETINE (Asclemed USA), DULOXETINE (Asclemed USA), DULOXETINE (Aurobindo Pharma Limited), DULOXETINE (BluePoint Laboratories), DULOXETINE (BluePoint Laboratories), DULOXETINE (BluePoint), DULOXETINE (Breckenridge Pharmaceutical), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Bryant Ranch Prepack), DULOXETINE (Camber Pharmaceuticals), DULOXETINE (Cardinal Health 107), DULOXETINE (Coupler LLC), DULOXETINE (Coupler LLC), DULOXETINE (Coupler LLC), DULOXETINE (Golden State Medical Supply), DULOXETINE (Golden State Medical Supply), DULOXETINE (Inventia Healthcare Private Limited), DULOXETINE (Lupin Pharmaceuticals), DULOXETINE (MARKSANS PHARMA LIMITED), DULOXETINE (Major Pharmaceuticals), DULOXETINE (NCS HealthCare of KY), DULOXETINE (Northwind Pharmaceuticals), DULOXETINE (NuCare Pharmaceuticals), DULOXETINE (NuCare Pharmaceuticals), DULOXETINE (NuCare Pharmaceuticals), DULOXETINE (NuCare Pharmaceuticals), DULOXETINE (NuCare Pharmaceuticals), DULOXETINE (PD-Rx Pharmaceuticals), DULOXETINE (PD-Rx Pharmaceuticals), DULOXETINE (PD-Rx Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Preferred Pharmaceuticals), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Proficient Rx LP), DULOXETINE (Quality Care Products LLC), DULOXETINE (Quality Care Products LLC), DULOXETINE (Quality Care Products), DULOXETINE (Quality Care Products), DULOXETINE (Quality Care Products), DULOXETINE (Quallent Pharmaceuticals Health LLC), DULOXETINE (Quallent Pharmaceuticals Health), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (REMEDYREPACK), DULOXETINE (Rising Pharma Holdings), DULOXETINE (ST. MARY'S MEDICAL PARK PHARMACY), DULOXETINE (ST. MARY'S MEDICAL PARK PHARMACY), DULOXETINE (ST. MARY'S MEDICAL PARK PHARMACY), DULOXETINE (ST. MARY'S MEDICAL PARK PHARMACY), DULOXETINE (Solco Healthcare US), DULOXETINE (Sun Pharmaceutical Industries), DULOXETINE (Zydus Lifesciences Limited), DULOXETINE D/R (Direct_Rx), DULOXETINE D/R (Direct_Rx), DULOXETINE HCL (Advanced Rx Pharmacy of Tennessee), DULOXETINE HCL (Advanced Rx of Tennessee), DULOXETINE HCL (Advanced Rx of Tennessee), DULOXETINE HCL (Advanced Rx of Tennessee), DULOXETINE HCL (Advanced Rx of Tennessee), DULOXETINE HYDROCHLORIDE (Alembic Pharmaceuticals Limited), DULOXETINE HYDROCHLORIDE (Macleods Pharmaceuticals Limited), DULOXETINE HYDROCHLORIDE (Proficient Rx LP), DULOXETINE HYDROCHLORIDE (Torrent Pharmaceuticals Limited), DULOXETINE HYDROCHLORIDE (Yaopharma), NA (Qingdao BAHEAL Pharmaceutical) |
|---|
| 組成式 |
C18H19NOS. HCl
|
|---|
| 質量 |
333.0954
|
|---|
| 分子量 |
333.88
|
|---|
| 構造式 |
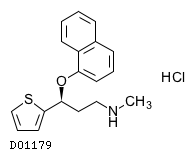
|
|---|
| Simcomp |
|
|---|
| クラス |
|
|---|
| コード |
| 商品 (DG00962): | D01179<JP/US> |
|
|---|
| 効能 |
抗うつ薬, セロトニン・ノルアドレナリン再取り込み阻害薬 |
|---|
| 疾患 |
|
|---|
| コメント |
フルオキセチン誘導体
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 代謝 |
Enzyme: CYP1A2 [HSA: 1544]; CYP2D6 [HSA: 1565]
|
|---|
| 相互作用 |
CYP inhibition: CYP2D6 [HSA: 1565]
|
|---|
| 構造マップ |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
1 神経系及び感覚器官用医薬品
11 中枢神経系用薬
117 精神神経用剤
1179 その他の精神神経用剤
D01179 塩酸デュロキセチン (JAN); デュロキセチン塩酸塩
119 その他の中枢神経系用薬
1190 その他の中枢神経系用薬
D01179 塩酸デュロキセチン (JAN); デュロキセチン塩酸塩
医療用医薬品のATC分類 [BR:jp08303]
N 神経系
N06 精神賦活薬
N06A 抗うつ薬
N06AX その他の抗うつ薬
N06AX21 デュロキセチン
D01179 塩酸デュロキセチン (JAN) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
抗うつ薬
SSRIs/SNRIs(選択的セロトニン/ノルアドレナリン再取り込み阻害薬)
デュロキセチン
D01179 塩酸デュロキセチン (JAN)
抗不安薬
SSRIs/SNRIs(選択的セロトニン/ノルアドレナリン再取り込み阻害薬)
デュロキセチン
D01179 塩酸デュロキセチン (JAN)
中枢神経系用薬
線維筋痛症治療薬
デュロキセチン
D01179 塩酸デュロキセチン (JAN)
医薬品グループ [BR:jp08330]
精神神経系用薬
DG01658 セロトニン・ノルアドレナリン再取り込み阻害薬 (SNRI)
DG00962 デュロキセチン
D01179 塩酸デュロキセチン
代謝酵素基質薬
DG01892 CYP1A2基質薬
DG00962 デュロキセチン
D01179 塩酸デュロキセチン
DG01644 CYP2D6基質薬
DG00962 デュロキセチン
D01179 塩酸デュロキセチン
代謝酵素阻害薬
DG01645 CYP2D6阻害薬
DG00962 デュロキセチン
D01179 塩酸デュロキセチン
ターゲットに基づく医薬品分類 [BR:jp08310]
トランスポーター
SLC ファミリー
SLC6
SLC6A2 (NAT1)
D01179 塩酸デュロキセチン (JAN) <JP/US>
SLC6A4 (HTT)
D01179 塩酸デュロキセチン (JAN) <JP/US>
日本の新薬 [jp08318.html]
新有効成分含有医薬品
D01179
日本・米国・欧州の新薬 [jp08328.html]
日米欧での承認状況
D01179
薬物代謝酵素とトランスポーター [jp08309.html]
薬物代謝酵素
D01179
ゲノムバイオマーカー [jp08341.html]
医薬品応答に影響を与える多型と変異
D01179
医薬品グループ [BR:jp08330]
精神神経系用薬
DG01658 セロトニン・ノルアドレナリン再取り込み阻害薬 (SNRI)
DG00962 デュロキセチン
代謝酵素基質薬
DG01892 CYP1A2基質薬
DG00962 デュロキセチン
DG01644 CYP2D6基質薬
DG00962 デュロキセチン
代謝酵素阻害薬
DG01645 CYP2D6阻害薬
DG00962 デュロキセチン
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|