| Entry |
|
|---|
| Name |
Methylprednisolone sodium succinate (JAN/USP);
A-methapred (TN);
Solu-medrol (TN) |
|---|
| Product |
SOLU-MEDROL (A-S Medication Solutions), SOLU-MEDROL (Cardinal Health 107), SOLU-MEDROL (Cardinal Health 107), SOLU-MEDROL (Cardinal Health 107), SOLU-MEDROL (HF Acquisition Co. LLC), SOLU-MEDROL (HF Acquisition Co. LLC), SOLU-MEDROL (Henry Schein), SOLU-MEDROL (Henry Schein), SOLU-MEDROL (Medical Purchasing Solutions), SOLU-MEDROL (Medical Purchasing Solutions), SOLU-MEDROL (Medical Purchasing Solutions), SOLU-MEDROL (Pharmacia & Upjohn Company LLC), SOLU-MEDROL (Pharmacia & Upjohn Company LLC), SOLU-MEDROL (ProPharma Distribution), SOLU-MEDROL (REMEDYREPACK), SOLU-MEDROL(R) METHYLPREDNISOLONE SODIUM SUCCINAT (HF Acquisition Co LLC) |
|---|
| Generic |
|
|---|
| Formula |
C26H33O8. Na
|
|---|
| Exact mass |
496.2073
|
|---|
| Mol weight |
496.52
|
|---|
| Structure |
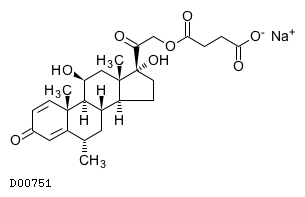
|
|---|
| Simcomp |
|
|---|
| Class |
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
|
|---|
| Remark |
| Therapeutic category: | 2456 |
| Product (DG00410): | D00407<JP/US> D00751<JP/US> D00979<JP/US> |
|
|---|
| Efficacy |
Anti-inflammatory, Immunosuppressant, Glucocorticoid receptor agonist |
|---|
| Disease |
Asthma [DS: H00079] Atopic dermatitis [DS: H01358] Contact dermatitis [DS: H01357] Allergic rhinitis [DS: H01360] Dermatitis herpetiformis [DS: H01362] Mycosis fungoides [DS: H01463] Pemphigus [DS: H01648] Erythema multiforme [DS: H01695] Stevens-Johnson syndrome [DS: H01694] Congenital adrenal hyperplasia [DS: H00216] Ulcerative colitis [DS: H01466] Acquired (autoimmune) hemolytic anemia [DS: H01585] Congenital (erythroid) hypoplastic anemia; Diamond-Blackfan anemia [DS: H00237] Idiopathic thrombocytopenic purpura [DS: H01240] Pure red cell aplasia [DS: H01586] Multiple sclerosis [DS: H01490] Ankylosing spondylitis [DS: H01674] Psoriatic arthritis [DS: H01507] Rheumatoid arthritis [DS: H00630] Juvenile idiopathic arthritis [DS: H01672] Dermatomyositis [DS: H01604] Temporal arteritis [DS: H01698] Polymyositis [DS: H01604] Systemic lupus erythematosus [DS: H00080] |
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
|
|---|
| Metabolism |
Enzyme: CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
|
|---|
| Structure map |
| map07225 | Glucocorticoid and mineralocorticoid receptor agonists/antagonists |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
D DERMATOLOGICALS
D07 CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
D07A CORTICOSTEROIDS, PLAIN
D07AA Corticosteroids, weak (group I)
D07AA01 Methylprednisolone
D00751 Methylprednisolone sodium succinate (JAN/USP) <JP/US>
D10 ANTI-ACNE PREPARATIONS
D10A ANTI-ACNE PREPARATIONS FOR TOPICAL USE
D10AA Corticosteroids, combinations for treatment of acne
D10AA02 Methylprednisolone
D00751 Methylprednisolone sodium succinate (JAN/USP) <JP/US>
H SYSTEMIC HORMONAL PREPARATIONS, EXCL. SEX HORMONES AND INSULINS
H02 CORTICOSTEROIDS FOR SYSTEMIC USE
H02A CORTICOSTEROIDS FOR SYSTEMIC USE, PLAIN
H02AB Glucocorticoids
H02AB04 Methylprednisolone
D00751 Methylprednisolone sodium succinate (JAN/USP) <JP/US>
USP drug classification [BR:br08302]
Hormonal Agents, Stimulant/Replacement/Modifying (Adrenal)
Methylprednisolone
D00751 Methylprednisolone sodium succinate (JAN/USP)
Inflammatory Bowel Disease Agents
Glucocorticoids
Methylprednisolone
D00751 Methylprednisolone sodium succinate (JAN/USP)
Therapeutic category of drugs in Japan [BR:br08301]
2 Agents affecting individual organs
24 Hormones
245 Adrenal hormone preparations
2456 Prednisolones
D00751 Methylprednisolone sodium succinate (JAN/USP)
Drug groups [BR:br08330]
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
DG00410 Methylprednisolone
D00751 Methylprednisolone sodium succinate
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00410 Methylprednisolone
D00751 Methylprednisolone sodium succinate
Drug classes [BR:br08332]
Anti-inflammatory
DG01955 Corticosteroid
D00751 Methylprednisolone sodium succinate
Target-based classification of drugs [BR:br08310]
Nuclear receptors
Estrogen like receptors
3-Ketosteroid receptor
NR3C1 (GR)
D00751 Methylprednisolone sodium succinate (JAN/USP) <JP/US>
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00751
Drug groups [BR:br08330]
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
DG00410 Methylprednisolone
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00410 Methylprednisolone
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 35
1 C2x C 26.6700 -18.6900
2 C5x C 26.6700 -20.0900
3 C2x C 27.8600 -20.7900
4 C2y C 29.1200 -20.0900
5 C1z C 29.1200 -18.6900
6 C2x C 27.8600 -17.9900
7 C1y C 30.3100 -20.7900
8 C1x C 31.5000 -20.0900
9 C1y C 31.5000 -18.6900
10 C1y C 30.3100 -17.9900
11 C1y C 32.7600 -17.9900
12 C1z C 32.7600 -16.5900
13 C1x C 31.5000 -15.8900
14 C1y C 30.3100 -16.5900
15 C1x C 35.1400 -17.9900
16 C1x C 35.1400 -16.5900
17 C1z C 33.9500 -15.8900
18 O5x O 25.4800 -20.7900
19 C1a C 29.1200 -17.2900
20 O1a O 29.1200 -15.8900
21 C1a C 32.7600 -15.1900
22 C5a C 33.9500 -14.2100
23 C1a C 30.3100 -22.1900
24 O1a O 35.1400 -15.1900
25 C1b C 35.1400 -13.5100
26 O5a O 32.7600 -13.5100
27 O7a O 36.3300 -14.2100
28 C7a C 37.5200 -13.5100
29 C1b C 38.7100 -14.2100
30 O6a O 37.5200 -12.1100
31 C1b C 39.9281 -13.5199
32 C6a C 41.1095 -14.2150
33 O6a O 42.3149 -13.5320 #-
34 O6a O 41.0981 -15.6100
35 Z Na 43.7500 -13.5100 #+
BOND 37
1 1 2 1
2 2 3 1
3 3 4 2
4 4 5 1
5 5 6 1
6 1 6 2
7 4 7 1
8 7 8 1
9 8 9 1
10 9 10 1
11 5 10 1
12 9 11 1
13 11 12 1
14 12 13 1
15 13 14 1
16 10 14 1
17 11 15 1
18 15 16 1
19 16 17 1
20 12 17 1
21 2 18 2
22 5 19 1 #Up
23 14 20 1 #Up
24 12 21 1 #Up
25 17 22 1 #Up
26 7 23 1 #Down
27 17 24 1 #Down
28 22 25 1
29 22 26 2
30 25 27 1
31 27 28 1
32 28 29 1
33 28 30 2
34 29 31 1
35 31 32 1
36 32 33 1
37 32 34 2
|
|---|