| Entry |
|
|---|
| Name |
Dexamethasone sodium phosphate (JAN/USP);
Dalalone (TN);
Maxidex (TN) |
|---|
| Product |
|
|---|
| Generic |
DEXAMETHASONE SODIUM PHOSPHATE (A-S Medication Solutions), DEXAMETHASONE SODIUM PHOSPHATE (A-S Medication Solutions), DEXAMETHASONE SODIUM PHOSPHATE (A-S Medication Solutions), DEXAMETHASONE SODIUM PHOSPHATE (A-S Medication Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Advanced Rx Pharmacy of Tennessee), DEXAMETHASONE SODIUM PHOSPHATE (Amneal Pharmaceuticals LLC), DEXAMETHASONE SODIUM PHOSPHATE (Amneal Pharmaceuticals LLC), DEXAMETHASONE SODIUM PHOSPHATE (Armas Pharmaceuticals), DEXAMETHASONE SODIUM PHOSPHATE (Asclemed USA), DEXAMETHASONE SODIUM PHOSPHATE (Asclemed USA), DEXAMETHASONE SODIUM PHOSPHATE (Asclemed USA), DEXAMETHASONE SODIUM PHOSPHATE (Asclemed USA), DEXAMETHASONE SODIUM PHOSPHATE (Bausch & Lomb Incorporated), DEXAMETHASONE SODIUM PHOSPHATE (Cardinal Health 107), DEXAMETHASONE SODIUM PHOSPHATE (Cardinal Health 107), DEXAMETHASONE SODIUM PHOSPHATE (Civica), DEXAMETHASONE SODIUM PHOSPHATE (Eugia US LLC), DEXAMETHASONE SODIUM PHOSPHATE (Eugia US LLC), DEXAMETHASONE SODIUM PHOSPHATE (Eugia US LLC), DEXAMETHASONE SODIUM PHOSPHATE (Fresenius Kabi USA), DEXAMETHASONE SODIUM PHOSPHATE (Fresenius Kabi USA), DEXAMETHASONE SODIUM PHOSPHATE (Fresenius Kabi USA), DEXAMETHASONE SODIUM PHOSPHATE (Fresenius Kabi USA), DEXAMETHASONE SODIUM PHOSPHATE (Fresenius Kabi USA), DEXAMETHASONE SODIUM PHOSPHATE (Fresenius Kabi USA), DEXAMETHASONE SODIUM PHOSPHATE (Fresenius Kabi USA), DEXAMETHASONE SODIUM PHOSPHATE (Gland Pharma Limited), DEXAMETHASONE SODIUM PHOSPHATE (HF Acquisition Co LLC), DEXAMETHASONE SODIUM PHOSPHATE (HF Acquisition Co LLC), DEXAMETHASONE SODIUM PHOSPHATE (HF Acquisition Co LLC), DEXAMETHASONE SODIUM PHOSPHATE (HF Acquisition Co LLC), DEXAMETHASONE SODIUM PHOSPHATE (HF Acquisition Co LLC), DEXAMETHASONE SODIUM PHOSPHATE (HF Acquisition Co LLC), DEXAMETHASONE SODIUM PHOSPHATE (HF Acquisition Co LLC), DEXAMETHASONE SODIUM PHOSPHATE (Henry Schein), DEXAMETHASONE SODIUM PHOSPHATE (Henry Schein), DEXAMETHASONE SODIUM PHOSPHATE (Henry Schein), DEXAMETHASONE SODIUM PHOSPHATE (Henry Schein), DEXAMETHASONE SODIUM PHOSPHATE (Hikma Pharmaceuticals USA), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Medical Purchasing Solutions), DEXAMETHASONE SODIUM PHOSPHATE (Micro Labs Limited), DEXAMETHASONE SODIUM PHOSPHATE (Mylan Institutional LLC), DEXAMETHASONE SODIUM PHOSPHATE (Mylan Institutional LLC), DEXAMETHASONE SODIUM PHOSPHATE (Phlow Corporation), DEXAMETHASONE SODIUM PHOSPHATE (ProPharma Distribution), DEXAMETHASONE SODIUM PHOSPHATE (REMEDYREPACK), DEXAMETHASONE SODIUM PHOSPHATE (REMEDYREPACK), DEXAMETHASONE SODIUM PHOSPHATE (REMEDYREPACK), DEXAMETHASONE SODIUM PHOSPHATE (Sagent Pharmaceuticals), DEXAMETHASONE SODIUM PHOSPHATE (Somerset Therapeutics), DEXAMETHASONE SODIUM PHOSPHATE (Somerset Therapeutics), DEXAMETHASONE SODIUM PHOSPHATE (Somerset Therapeutics) |
|---|
| Formula |
C22H28FO8P. 2Na
|
|---|
| Exact mass |
516.1301
|
|---|
| Mol weight |
516.40
|
|---|
| Structure |
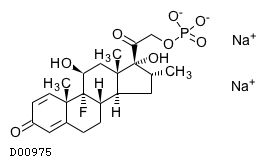
|
|---|
| Simcomp |
|
|---|
| Class |
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
Dermatological agent
DG03243 Atopic dermatitis agent
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
Metabolizing enzyme inducer
DG02853 CYP3A/CYP3A4 inducer
DG01635 CYP3A4 inducer
|
|---|
| Remark |
| Product (DG00011): | D00292<JP/US> D00975<JP/US> D01510<JP> D01615<JP> D01632<JP> D01948<JP> D07073<JP> |
|
|---|
| Efficacy |
Anti-inflammatory, Glucocorticoid receptor agonist |
|---|
| Disease |
Primary or secondary adrenocortical insufficiency [DS: H01598] Congenital adrenal hyperplasia [DS: H00216] Rheumatoid arthritis [DS: H00630] Juvenile rheumatoid arthritis [DS: H01672] Acute gouty arthritis [DS: H01532] Psoriatic arthritis [DS: H01507] Ankylosing spondylitis [DS: H01674] Systemic lupus erythematosus [DS: H00080] Pemphigus [DS: H01648] Severe erythema multiforme [DS: H01695] Stevens-Johnson syndrome [DS: H01694] Seborrheic dermatitis [DS: H01652] Psoriasis [DS: H01656] Mycosis fungoides [DS: H01463] Bronchial asthma [DS: H00079] Contact dermatitis [DS: H01357] Atopic dermatitis [DS: H01358] Seasonal or perennial allergic rhinitis [DS: H01360] Herpes zoster ophthalmicus [DS: H00366] Optic neuritis [DS: H01717] Ulcerative colitis [DS: H01466] Pulmonary tuberculosis [DS: H00342] Acquired (autoimmune) hemolytic anemia [DS: H01585] Nephrotic syndrome [DS: H01657] |
|---|
| Target |
|
|---|
| Pathway |
| hsa04080 | Neuroactive ligand-receptor interaction |
|
|---|
| Metabolism |
Enzyme: CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
CYP induction: CYP3A4 [HSA: 1576]
|
|---|
| Structure map |
| map07225 | Glucocorticoid and mineralocorticoid receptor agonists/antagonists |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
A ALIMENTARY TRACT AND METABOLISM
A01 STOMATOLOGICAL PREPARATIONS
A01A STOMATOLOGICAL PREPARATIONS
A01AC Corticosteroids for local oral treatment
A01AC02 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
C CARDIOVASCULAR SYSTEM
C05 VASOPROTECTIVES
C05A AGENTS FOR TREATMENT OF HEMORRHOIDS AND ANAL FISSURES FOR TOPICAL USE
C05AA Corticosteroids
C05AA09 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
D DERMATOLOGICALS
D07 CORTICOSTEROIDS, DERMATOLOGICAL PREPARATIONS
D07A CORTICOSTEROIDS, PLAIN
D07AB Corticosteroids, moderately potent (group II)
D07AB19 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
D07X CORTICOSTEROIDS, OTHER COMBINATIONS
D07XB Corticosteroids, moderately potent, other combinations
D07XB05 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
D10 ANTI-ACNE PREPARATIONS
D10A ANTI-ACNE PREPARATIONS FOR TOPICAL USE
D10AA Corticosteroids, combinations for treatment of acne
D10AA03 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
H SYSTEMIC HORMONAL PREPARATIONS, EXCL. SEX HORMONES AND INSULINS
H02 CORTICOSTEROIDS FOR SYSTEMIC USE
H02A CORTICOSTEROIDS FOR SYSTEMIC USE, PLAIN
H02AB Glucocorticoids
H02AB02 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
R RESPIRATORY SYSTEM
R01 NASAL PREPARATIONS
R01A DECONGESTANTS AND OTHER NASAL PREPARATIONS FOR TOPICAL USE
R01AD Corticosteroids
R01AD03 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
S SENSORY ORGANS
S01 OPHTHALMOLOGICALS
S01B ANTIINFLAMMATORY AGENTS
S01BA Corticosteroids, plain
S01BA01 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
S01C ANTIINFLAMMATORY AGENTS AND ANTIINFECTIVES IN COMBINATION
S01CB Corticosteroids/antiinfectives/mydriatics in combination
S01CB01 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
S02 OTOLOGICALS
S02B CORTICOSTEROIDS
S02BA Corticosteroids
S02BA06 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
S03 OPHTHALMOLOGICAL AND OTOLOGICAL PREPARATIONS
S03B CORTICOSTEROIDS
S03BA Corticosteroids
S03BA01 Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
USP drug classification [BR:br08302]
Hormonal Agents, Stimulant/Replacement/Modifying (Adrenal)
Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP)
Inflammatory Bowel Disease Agents
Glucocorticoids
Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP)
Ophthalmic Agents
Ophthalmic Anti-inflammatories
Glucocorticoids, Ophthalmic
Dexamethasone
D00975 Dexamethasone sodium phosphate (JAN/USP)
Therapeutic category of drugs in Japan [BR:br08301]
1 Agents affecting nervous system and sensory organs
13 Agents affecting sensory organs
131 Ophthalmic agents
1315 Ophthalmic cortisones
D00975 Dexamethasone sodium phosphate (JAN/USP)
132 Otic and nasal agents
1329 Others
D00975 Dexamethasone sodium phosphate (JAN/USP)
2 Agents affecting individual organs
24 Hormones
245 Adrenal hormone preparations
2454 Fluorinated adrenocorticotropic hormones
D00975 Dexamethasone sodium phosphate (JAN/USP)
Drug groups [BR:br08330]
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
DG00011 Dexamethasone
D00975 Dexamethasone sodium phosphate
Dermatological agent
DG03243 Atopic dermatitis agent
D00975 Dexamethasone sodium phosphate
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00011 Dexamethasone
D00975 Dexamethasone sodium phosphate
Metabolizing enzyme inducer
DG02853 CYP3A/CYP3A4 inducer
DG01635 CYP3A4 inducer
DG00011 Dexamethasone
D00975 Dexamethasone sodium phosphate
Drug classes [BR:br08332]
Anti-inflammatory
DG01955 Corticosteroid
D00975 Dexamethasone sodium phosphate
Dermatological agent
DG03243 Atopic dermatitis agent
D00975 Dexamethasone sodium phosphate
Target-based classification of drugs [BR:br08310]
Nuclear receptors
Estrogen like receptors
3-Ketosteroid receptor
NR3C1 (GR)
D00975 Dexamethasone sodium phosphate (JAN/USP) <JP/US>
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00975
Drug groups [BR:br08330]
Anti-inflammatory
DG01955 Corticosteroid
DG02068 Glucocorticoid
DG00011 Dexamethasone
Metabolizing enzyme substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00011 Dexamethasone
Metabolizing enzyme inducer
DG02853 CYP3A/CYP3A4 inducer
DG01635 CYP3A4 inducer
DG00011 Dexamethasone
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 34
1 C1y C 20.5509 -18.6771
2 C1y C 19.3622 -19.3588
3 C1z C 20.5626 -17.2961
4 C1x C 22.9399 -18.7004
5 C1z C 18.1794 -18.6655
6 C1x C 19.3622 -20.7281
7 C1z C 21.7686 -16.6144
8 C1x C 19.3855 -16.6144
9 C1a C 20.5567 -15.9269
10 C1y C 22.9514 -17.3253
11 C1z C 16.9908 -19.3471
12 C1y C 18.1910 -17.2961
13 X F 18.1677 -20.0289
14 C1x C 18.1677 -21.4098
15 C5a C 21.7745 -15.2451
16 O1a O 23.0913 -16.2589
17 C1a C 24.2741 -17.1681
18 C2y C 16.9849 -20.7222
19 C2x C 15.8021 -18.6596
20 C1a C 16.9791 -17.9662
21 O1a O 17.0025 -16.6028
22 C1b C 22.9573 -14.5575
23 O5a O 20.5743 -14.5575
24 C2x C 15.8021 -21.4040
25 C2x C 14.6135 -19.3471
26 O2b O 24.1401 -15.2334
27 C5x C 14.6135 -20.7222
28 P1b P 25.2763 -14.4585
29 O5x O 13.4190 -21.3981
30 O1c O 26.4534 -15.1461
31 O1c O 26.4068 -13.6836 #-
32 O1c O 24.5014 -13.3223 #-
33 Z Na 28.9880 -14.9538 #+
34 Z Na 28.9181 -18.1935 #+
BOND 35
1 1 2 1
2 1 3 1
3 1 4 1
4 2 5 1
5 2 6 1
6 3 7 1
7 3 8 1
8 3 9 1 #Up
9 4 10 1
10 5 11 1
11 5 12 1
12 5 13 1 #Down
13 6 14 1
14 7 15 1 #Up
15 7 16 1 #Down
16 10 17 1 #Down
17 11 18 1
18 11 19 1
19 11 20 1 #Up
20 12 21 1 #Up
21 15 22 1
22 15 23 2
23 18 24 2
24 19 25 2
25 22 26 1
26 24 27 1
27 26 28 1
28 27 29 2
29 28 30 2
30 28 31 1
31 28 32 1
32 7 10 1
33 8 12 1
34 14 18 1
35 25 27 1
|
|---|
|