 | | DRUG: ゾルピデム酒石酸塩 | |
| エントリ |
|
|---|
| 一般名 |
ゾルピデム酒石酸塩 (JP18);
Zolpidem tartrate (JP18/USP) |
|---|
| 商品名 |
|
|---|
| 後発品 |
ゾルピデム酒石酸塩 (Meiji Seikaファルマ), ゾルピデム酒石酸塩 (アルフレッサファーマ), ゾルピデム酒石酸塩 (キョーリンリメディオ), ゾルピデム酒石酸塩 (サンド), ゾルピデム酒石酸塩 (ニプロ), ゾルピデム酒石酸塩 (全星薬品工業), ゾルピデム酒石酸塩 (共創未来ファーマ), ゾルピデム酒石酸塩 (共和薬品工業), ゾルピデム酒石酸塩 (大原薬品工業), ゾルピデム酒石酸塩 (大原薬品工業), ゾルピデム酒石酸塩 (救急薬品工業), ゾルピデム酒石酸塩 (日医工), ゾルピデム酒石酸塩 (日医工), ゾルピデム酒石酸塩 (日医工岐阜工場), ゾルピデム酒石酸塩 (日新製薬-山形), ゾルピデム酒石酸塩 (日新製薬-山形), ゾルピデム酒石酸塩 (日本ケミファ), ゾルピデム酒石酸塩 (日本ケミファ), ゾルピデム酒石酸塩 (日本ジェネリック), ゾルピデム酒石酸塩 (東和薬品), ゾルピデム酒石酸塩 (東洋カプセル), ゾルピデム酒石酸塩 (沢井製薬), ゾルピデム酒石酸塩 (皇漢堂製薬), ゾルピデム酒石酸塩 (第一三共エスファ), ゾルピデム酒石酸塩 (辰巳化学), ゾルピデム酒石酸塩 (陽進堂), ゾルピデム酒石酸塩 (高田製薬), ゾルピデム酒石酸塩 (高田製薬) |
|---|
| 米国の商品 |
|
|---|
| 後発品 |
ZOLPIDEM (ACI Healthcare USA), ZOLPIDEM (Advanced Rx of Tennessee), ZOLPIDEM (Aphena Pharma Solutions - Tennessee), ZOLPIDEM (Aphena Pharma Solutions - Tennessee), ZOLPIDEM (Aphena Pharma Solutions - Tennessee), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Bryant Ranch Prepack), ZOLPIDEM (Golden State Medical Supply), ZOLPIDEM (Proficient Rx LP), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (A-S Medication Solutions), ZOLPIDEM TARTRATE (Advanced Rx Pharmacy of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx Pharmacy of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx Pharmacy of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx Pharmacy of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx of Tennessee), ZOLPIDEM TARTRATE (Advanced Rx of Tennessee), ZOLPIDEM TARTRATE (American Health Packaging), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Aphena Pharma Solutions - Tennessee), ZOLPIDEM TARTRATE (Asclemed USA), ZOLPIDEM TARTRATE (Asclemed USA), ZOLPIDEM TARTRATE (Asclemed USA), ZOLPIDEM TARTRATE (Asclemed USA), ZOLPIDEM TARTRATE (Aurobindo Pharma Limited), ZOLPIDEM TARTRATE (AvKARE), ZOLPIDEM TARTRATE (Breckenridge Pharmaceutical), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Bryant Ranch Prepack), ZOLPIDEM TARTRATE (Calvin Scott &), ZOLPIDEM TARTRATE (Cardinal Health 107), ZOLPIDEM TARTRATE (Chartwell RX), ZOLPIDEM TARTRATE (DIRECT RX), ZOLPIDEM TARTRATE (DirectRX), ZOLPIDEM TARTRATE (Direct_Rx), ZOLPIDEM TARTRATE (Golden State Medical Supply), ZOLPIDEM TARTRATE (Lake Erie Medical DBA Quality Care Products LLC), ZOLPIDEM TARTRATE (Lupin Pharmaceuticals), ZOLPIDEM TARTRATE (Lupin Pharmaceuticals), ZOLPIDEM TARTRATE (Major Pharmaceuticals), ZOLPIDEM TARTRATE (NorthStar Rx LLC), ZOLPIDEM TARTRATE (Northwind Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (NuCare Pharmaceuticals), ZOLPIDEM TARTRATE (PD-Rx Pharmaceuticals), ZOLPIDEM TARTRATE (PD-Rx Pharmaceuticals), ZOLPIDEM TARTRATE (Preferred Pharmaceuticals), ZOLPIDEM TARTRATE (Preferred Pharmaceuticals), ZOLPIDEM TARTRATE (Preferred Pharmaceuticals), ZOLPIDEM TARTRATE (Preferred Pharmaceuticals), ZOLPIDEM TARTRATE (Preferred Pharmaceuticals), ZOLPIDEM TARTRATE (Preferred Pharmaceuticals), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Proficient Rx LP), ZOLPIDEM TARTRATE (Quality Care Products LLC), ZOLPIDEM TARTRATE (Quality Care Products), ZOLPIDEM TARTRATE (REMEDYREPACK), ZOLPIDEM TARTRATE (REMEDYREPACK), ZOLPIDEM TARTRATE (REMEDYREPACK), ZOLPIDEM TARTRATE (ST. MARY'S MEDICAL PARK PHARMACY), ZOLPIDEM TARTRATE (ST. MARY'S MEDICAL PARK PHARMACY), ZOLPIDEM TARTRATE (Sandoz), ZOLPIDEM TARTRATE (Sandoz), ZOLPIDEM TARTRATE (Sanofi-Aventis U.S. LLC), ZOLPIDEM TARTRATE (St Marys Medical Park Pharmacy), ZOLPIDEM TARTRATE (St. Mary's Medical Park Pharmacy), ZOLPIDEM TARTRATE (Sun Pharmaceutical Industries), ZOLPIDEM TARTRATE (Teva Pharmaceuticals USA), ZOLPIDEM TARTRATE (The ACME Laboratories), ZOLPIDEM TARTRATE (Torrent Pharmaceuticals Limited), ZOLPIDEM TARTRATE SUBLINGUAL (ENDO USA) |
|---|
| 組成式 |
(C19H21N3O)2. C4H6O6
|
|---|
| 質量 |
764.3534
|
|---|
| 分子量 |
764.86
|
|---|
| 構造式 |
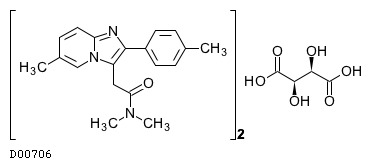
|
|---|
| クラス |
|
|---|
| コード |
| 商品 (DG00922): | D00706<JP/US> |
|
|---|
| 効能 |
催眠鎮静薬 |
|---|
| 疾患 |
|
|---|
| コメント |
超短時間型非ベンゾジアゼピン系 (イミダゾピリジン誘導体)
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 代謝 |
Enzyme: CYP3A4 [HSA: 1576]; CYP2C9 [HSA: 1559], CYP1A2 [HSA: 1544]
|
|---|
| 相互作用 |
|
|---|
| 構造マップ |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
1 神経系及び感覚器官用医薬品
11 中枢神経系用薬
112 催眠鎮静剤、抗不安剤
1129 その他の催眠鎮静剤、抗不安剤
D00706 ゾルピデム酒石酸塩 (JP18)
医療用医薬品のATC分類 [BR:jp08303]
N 神経系
N05 精神抑制薬
N05C 睡眠薬と鎮静薬
N05CF ベンゾジアゼピン関連薬物
N05CF02 ゾルピデム
D00706 ゾルピデム酒石酸塩 (JP18) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
睡眠障害治療薬
睡眠促進薬
GABA受容体複合体
ゾルピデム
D00706 ゾルピデム酒石酸塩 (JP18)
医薬品グループ [BR:jp08330]
精神神経系用薬
DG01836 非ベンゾジアゼピン系催眠鎮静薬
DG00922 ゾルピデム
D00706 ゾルピデム酒石酸塩
DG03202 睡眠薬
DG00922 ゾルピデム
D00706 ゾルピデム酒石酸塩
DG01567 GABA-A受容体作動薬
DG00922 ゾルピデム
D00706 ゾルピデム酒石酸塩
代謝酵素基質薬
DG01892 CYP1A2基質薬
DG00922 ゾルピデム
D00706 ゾルピデム酒石酸塩
DG01642 CYP2C9基質薬
DG00922 ゾルピデム
D00706 ゾルピデム酒石酸塩
DG01633 CYP3A/CYP3A4基質薬
DG02913 CYP3A4基質薬
DG00922 ゾルピデム
D00706 ゾルピデム酒石酸塩
医薬品クラス [BR:jp08332]
精神神経系用薬
DG03202 睡眠薬 [商品一覧]
D00706 ゾルピデム酒石酸塩
ターゲットに基づく医薬品分類 [BR:jp08310]
イオンチャネル
リガンド依存性イオンチャネル
GABA (イオンチャネル型)
GABR
D00706 ゾルピデム酒石酸塩 (JP18) <JP/US>
日本薬局方収載医薬品 [BR:jp08311]
化学薬品等
D00706 ゾルピデム酒石酸塩
D00706 ゾルピデム酒石酸塩錠
日本の新薬 [jp08318.html]
新有効成分含有医薬品
D00706
薬物代謝酵素とトランスポーター [jp08309.html]
薬物代謝酵素
D00706
医薬品グループ [BR:jp08330]
精神神経系用薬
DG01836 非ベンゾジアゼピン系催眠鎮静薬
DG00922 ゾルピデム
DG03202 睡眠薬
DG00922 ゾルピデム
DG01567 GABA-A受容体作動薬
DG00922 ゾルピデム
代謝酵素基質薬
DG01892 CYP1A2基質薬
DG00922 ゾルピデム
DG01642 CYP2C9基質薬
DG00922 ゾルピデム
DG01633 CYP3A/CYP3A4基質薬
DG02913 CYP3A4基質薬
DG00922 ゾルピデム
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|