| Entry |
|
|---|
| Name |
Lidocaine (JP18/USP/INN);
Dentipatch (TN);
Xylocaine (TN) |
|---|
| Product |
|
|---|
| Generic |
LIDOCAINE (A-S Medication Solutions), LIDOCAINE (AVKARE), LIDOCAINE (Actavis Pharma), LIDOCAINE (Actavis Pharma), LIDOCAINE (Advanced Rx Pharmacy of Tennessee), LIDOCAINE (Advanced Rx Pharmacy of Tennessee), LIDOCAINE (Advanced Rx Pharmacy of Tennessee), LIDOCAINE (Advanced Rx Pharmacy of Tennessee), LIDOCAINE (Advanced Rx Pharmacy of Tennessee), LIDOCAINE (Alembic Pharmaceuticals), LIDOCAINE (Amneal Pharmaceuticals LLC), LIDOCAINE (Amneal Pharmaceuticals LLC), LIDOCAINE (Amneal Pharmaceuticals LLC), LIDOCAINE (Ascend Laboratories), LIDOCAINE (Asclemed USA), LIDOCAINE (Asclemed USA), LIDOCAINE (Asclemed USA), LIDOCAINE (Asclemed USA), LIDOCAINE (Asclemed USA), LIDOCAINE (Aurobindo Pharma Limited), LIDOCAINE (AvKARE), LIDOCAINE (AvKARE), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Bryant Ranch Prepack), LIDOCAINE (Cardinal Health 107), LIDOCAINE (Cardinal Health 107), LIDOCAINE (DIRECT RX), LIDOCAINE (DIRECT RX), LIDOCAINE (DIRECTRX), LIDOCAINE (Direct_Rx), LIDOCAINE (Direct_Rx), LIDOCAINE (Direct_Rx), LIDOCAINE (Fougera Pharmaceuticals), LIDOCAINE (Glenmark Pharmaceuticals), LIDOCAINE (Golden State Medical Supply), LIDOCAINE (Golden State Medical Supply), LIDOCAINE (IPG PHARMACEUTICALS), LIDOCAINE (IPG Pharmaceuticals), LIDOCAINE (Lake Erie Medical DBA Quality Care Products LLC), LIDOCAINE (Macleods Pharmaceuticals Limited), LIDOCAINE (Mylan Pharmaceuticals), LIDOCAINE (Northstar Rx LLC), LIDOCAINE (NuCare Pharmaceuticals), LIDOCAINE (Par Pharmaceutical), LIDOCAINE (Preferred Pharmaceuticals), LIDOCAINE (Preferred Pharmaceuticals), LIDOCAINE (Preferred Pharmaceuticals), LIDOCAINE (Preferred Pharmaceuticals), LIDOCAINE (Preferred Pharmaceuticals), LIDOCAINE (Preferred Pharmaceuticals), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (Proficient Rx LP), LIDOCAINE (PureTek Corporation), LIDOCAINE (QUAGEN PHARMACEUTICALS LLC), LIDOCAINE (Quality Care Products LLC), LIDOCAINE (Quality Care Products), LIDOCAINE (Quality Care Products), LIDOCAINE (Quality Care; Products), LIDOCAINE (REMEDYREPACK), LIDOCAINE (REMEDYREPACK), LIDOCAINE (REMEDYREPACK), LIDOCAINE (REMEDYREPACK), LIDOCAINE (REMEDYREPACK), LIDOCAINE (REMEDYREPACK), LIDOCAINE (REMEDYREPACK), LIDOCAINE (REMEDYREPACK), LIDOCAINE (Septodont), LIDOCAINE (St. Mary's Medical Park Pharmacy), LIDOCAINE (St. Mary's Medical Park Pharmacy), LIDOCAINE (Strides Pharma Science Limited), LIDOCAINE (Taro Pharmaceuticals U.S.A.), LIDOCAINE (Taro Pharmaceuticals U.S.A.), LIDOCAINE (UNIT DOSE SERVICES), LIDOCAINE (Unit Dose Services), LIDOCAINE (medsource pharmaceuticals), LIDOCAINE (medsource pharmaceuticals), LIDOCAINE (medsource pharmaceuticals), LIDOCAINE (medsource pharmaceuticals), LIDOCAINE (medsource pharmaceuticals), LIDOCAINE (medsource pharmaceuticals), LIDOCAINE 5% (NuCare Pharmaceuticals), LIDOCAINE 5% (REMEDYREPACK), LIDOCAINE 5% (ST. MARY'S MEDICAL PARK PHARMACY), LIDOCAINE 5% (YARAL Pharma), LIDOCAINE PATCH (Direct_Rx), LIDOCAN (PURETEK CORPORATION), LIDOCAN III (PURETEK CORPORATION), LIDOCAN IV (PURETEK CORPORATION), LIDOCAN V (PURETEK CORPORATION), LMR PLUS (V2 Pharma), TRIDACAINE (Trifluent Pharma LLC), TRIDACAINE II (Trifluent Pharma LLC), TRIDACAINE III (Trifluent Pharma LLC) |
|---|
| Formula |
C14H22N2O
|
|---|
| Exact mass |
234.1732
|
|---|
| Mol weight |
234.34
|
|---|
| Structure |
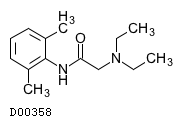
|
|---|
| Simcomp |
|
|---|
| Class |
Cardiovascular agent
DG01653 Antiarrhythmics
DG01652 Class I antiarrhythmic agent
DG01651 Class Ib antiarrhythmic agent
Neuropsychiatric agent
DG02030 Anesthetics
DG01675 Local anesthetic
DG01673 Amide type local anesthetic
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
|
|---|
| Remark |
| Therapeutic category: | 1214 |
| Product (DG00196): | D00358<JP/US> D02086<JP/US> D08127<JP/US> |
| Product (mixture): | D02740<JP/US> D04688<JP> D04692<JP> D04881<JP> D07742<JP> D11553<US> |
|
|---|
| Efficacy |
Anesthetic (topical), Antiarrhythmic, Sodium channel blocker |
|---|
| Comment |
Anilide derivative
|
|---|
| Target |
|
|---|
| Pathway |
| hsa04261 | Adrenergic signaling in cardiomyocytes |
|
|---|
| Metabolism |
Enzyme: CYP1A2 [HSA: 1544], CYP3A4 [HSA: 1576]
|
|---|
| Interaction |
|
|---|
| Structure map |
|
|---|
| Other map |
| map00982 | Drug metabolism - cytochrome P450 |
|
|---|
| Brite |
Anatomical Therapeutic Chemical (ATC) classification [BR:br08303]
C CARDIOVASCULAR SYSTEM
C01 CARDIAC THERAPY
C01B ANTIARRHYTHMICS, CLASS I AND III
C01BB Antiarrhythmics, class Ib
C01BB01 Lidocaine
D00358 Lidocaine (JP18/USP/INN) <JP/US>
C05 VASOPROTECTIVES
C05A AGENTS FOR TREATMENT OF HEMORRHOIDS AND ANAL FISSURES FOR TOPICAL USE
C05AD Local anesthetics
C05AD01 Lidocaine
D00358 Lidocaine (JP18/USP/INN) <JP/US>
D DERMATOLOGICALS
D04 ANTIPRURITICS, INCL. ANTIHISTAMINES, ANESTHETICS, ETC.
D04A ANTIPRURITICS, INCL. ANTIHISTAMINES, ANESTHETICS, ETC.
D04AB Anesthetics for topical use
D04AB01 Lidocaine
D00358 Lidocaine (JP18/USP/INN) <JP/US>
N NERVOUS SYSTEM
N01 ANESTHETICS
N01B ANESTHETICS, LOCAL
N01BB Amides
N01BB02 Lidocaine
D00358 Lidocaine (JP18/USP/INN) <JP/US>
R RESPIRATORY SYSTEM
R02 THROAT PREPARATIONS
R02A THROAT PREPARATIONS
R02AD Anesthetics, local
R02AD02 Lidocaine
D00358 Lidocaine (JP18/USP/INN) <JP/US>
S SENSORY ORGANS
S01 OPHTHALMOLOGICALS
S01H LOCAL ANESTHETICS
S01HA Local anesthetics
S01HA07 Lidocaine
D00358 Lidocaine (JP18/USP/INN) <JP/US>
S02 OTOLOGICALS
S02D OTHER OTOLOGICALS
S02DA Analgesics and anesthetics
S02DA01 Lidocaine
D00358 Lidocaine (JP18/USP/INN) <JP/US>
USP drug classification [BR:br08302]
Anesthetics
Local Anesthetics
Lidocaine
D00358 Lidocaine (JP18/USP/INN)
Therapeutic category of drugs in Japan [BR:br08301]
1 Agents affecting nervous system and sensory organs
12 Agents affecting peripheral nervous system
121 Local anesthetics
1214 Xylidines
D00358 Lidocaine (JP18/USP/INN)
Classification of Japanese OTC drugs [BR:br08313]
Agents for urogenital organs and anus
30 Hemorrhoid drugs for external use
D00358 Lidocaine (JP18/USP/INN)
Agents for integumentary system
57 Analgesic, antipruritic, astringent, and anti-inflammatory remedies (incl. gel patches)
D00358 Lidocaine (JP18/USP/INN)
Risk category of Japanese OTC drugs [BR:br08312]
Second-class OTC drugs
Inorganic and organic chemicals
Lidocaine
D00358 Lidocaine (JP18/USP/INN)
Drug groups [BR:br08330]
Cardiovascular agent
DG01653 Antiarrhythmics
DG01652 Class I antiarrhythmic agent
DG01651 Class Ib antiarrhythmic agent
DG00196 Lidocaine
D00358 Lidocaine
Neuropsychiatric agent
DG02030 Anesthetics
DG01675 Local anesthetic
DG01673 Amide type local anesthetic
DG00196 Lidocaine
D00358 Lidocaine
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00196 Lidocaine
D00358 Lidocaine
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00196 Lidocaine
D00358 Lidocaine
Drug classes [BR:br08332]
Cardiovascular agent
DG01653 Antiarrhythmics
D00358 Lidocaine
Target-based classification of drugs [BR:br08310]
Ion channels
Voltage-gated ion channels
Sodium channels
SCN1A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN2A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN3A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN4A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN5A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN8A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN9A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN10A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
SCN11A
D00358 Lidocaine (JP18/USP/INN) <JP/US>
Drugs listed in the Japanese Pharmacopoeia [BR:br08311]
Chemicals
D00358 Lidocaine
D00358 Lidocaine injection
Drug metabolizing enzymes and transporters [br08309.html]
Drug metabolizing enzymes
D00358
Drug groups [BR:br08330]
Cardiovascular agent
DG01653 Antiarrhythmics
DG01652 Class I antiarrhythmic agent
DG01651 Class Ib antiarrhythmic agent
DG00196 Lidocaine
Neuropsychiatric agent
DG02030 Anesthetics
DG01675 Local anesthetic
DG01673 Amide type local anesthetic
DG00196 Lidocaine
Metabolizing enzyme substrate
DG01892 CYP1A2 substrate
DG00196 Lidocaine
DG01633 CYP3A/CYP3A4 substrate
DG02913 CYP3A4 substrate
DG00196 Lidocaine
|
|---|
| Other DBs |
|
|---|
| LinkDB |
|
|---|
| KCF data |
ATOM 17
1 C8y C 23.2602 -19.0646
2 N1b N 24.4935 -19.7778
3 C8y C 22.0325 -19.7836
4 C8y C 23.2602 -17.6441
5 C5a C 25.7213 -19.0587
6 C8x C 20.8049 -19.0646
7 C1a C 22.0384 -21.1983
8 C8x C 22.0325 -16.9367
9 C1a C 24.4818 -16.9367
10 C1b C 26.9488 -19.7778
11 O5a O 25.7096 -17.6332
12 C8x C 20.8049 -17.6441
13 N1c N 28.1823 -19.0587
14 C1b C 29.4158 -19.7778
15 C1b C 28.1823 -17.6441
16 C1a C 30.6317 -19.0587
17 C1a C 29.4158 -16.9309
BOND 17
1 1 2 1
2 1 3 2
3 1 4 1
4 2 5 1
5 3 6 1
6 3 7 1
7 4 8 2
8 4 9 1
9 5 10 1
10 5 11 2
11 6 12 2
12 10 13 1
13 13 14 1
14 13 15 1
15 14 16 1
16 15 17 1
17 8 12 1
|
|---|
|