 | | DRUG: リドカイン塩酸塩 | |
| エントリ |
|
|---|
| 一般名 |
リドカイン塩酸塩 (JAN);
塩酸リドカイン;
Lidocaine hydrochloride (JAN/USP) |
|---|
| 商品名 |
|
|---|
| 後発品 |
|
|---|
| 米国の商品 |
AKTEN (Thea Pharma), LIDOCAINE (Fresenius Kabi USA), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI AND DEXTROSE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE AND DEXTROSE (Baxter Healthcare Corporation), LIDOCAINE HYDROCHLORIDE AND DEXTROSE (HF Acquisition Co LLC), XYLOCAIN (LIDOCAINE HCL ) (HF Acquisition Co LLC), XYLOCAINE (Fresenius Kabi USA), XYLOCAINE (Fresenius Kabi USA), XYLOCAINE (General Injectables & Vaccines), XYLOCAINE (General Injectables and Vaccines), XYLOCAINE (HF Acquisition Co LLC), XYLOCAINE (HF Acquisition Co LLC), XYLOCAINE (Henry Schein), XYLOCAINE MPF (HF Acquisition Co LLC), XYLOCAINE MPF (HF Acquisition Co LLC), XYLOCAINE MPF (HF Acquisition Co LLC), XYLOCAINE- MPF (LIDOCAINE HCL ) (HF Acquisition Co LLC) |
|---|
| 後発品 |
0.5% LIDOCAINE HCL (HF Acquisition Co LLC), GLYDO (Sagent Pharmaceuticals), LIDOCAINE (A-S Medication Solutions), LIDOCAINE (ARMAS PHARMACEUTICALS), LIDOCAINE (Fresenius Kabi USA), LIDOCAINE (Hikma Pharmaceuticals USA), LIDOCAINE (Medical Purchasing Solutions), LIDOCAINE (Medical Purchasing Solutions), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCI (HF Acquisition Co LLC), LIDOCAINE HCL (HF Acquisition Co LLC), LIDOCAINE HCL (HF Acquisition Co LLC), LIDOCAINE HCL (Nextsource Pharma), LIDOCAINE HCL (RXnSupplies Corporation), LIDOCAINE HYDROCHLORIDE (A-S Medication Solutions), LIDOCAINE HYDROCHLORIDE (A-S Medication Solutions), LIDOCAINE HYDROCHLORIDE (ATLANTIC BIOLOGICALS CORP.), LIDOCAINE HYDROCHLORIDE (ATLANTIC BIOLOGICALS CORP.), LIDOCAINE HYDROCHLORIDE (Advagen Pharma), LIDOCAINE HYDROCHLORIDE (Advanced Rx Pharmacy of Tennessee), LIDOCAINE HYDROCHLORIDE (Afaxys Pharma LLC), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (Asclemed USA), LIDOCAINE HYDROCHLORIDE (B. Braun Medical), LIDOCAINE HYDROCHLORIDE (Brookfield Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (Bryant Ranch Prepack), LIDOCAINE HYDROCHLORIDE (Bryant Ranch Prepack), LIDOCAINE HYDROCHLORIDE (Bryant Ranch Prepack), LIDOCAINE HYDROCHLORIDE (Camber Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (Camber Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (Cardinal Health 107), LIDOCAINE HYDROCHLORIDE (Cardinal Health 107), LIDOCAINE HYDROCHLORIDE (Chartwell RX), LIDOCAINE HYDROCHLORIDE (Civica), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (Eugia US LLC), LIDOCAINE HYDROCHLORIDE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE (HF Acquisition Co LLC), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Henry Schein), LIDOCAINE HYDROCHLORIDE (Hikma Pharmaceuticals USA), LIDOCAINE HYDROCHLORIDE (Hikma Pharmaceuticals USA), LIDOCAINE HYDROCHLORIDE (Hospira), LIDOCAINE HYDROCHLORIDE (Huons), LIDOCAINE HYDROCHLORIDE (International Medication Systems), LIDOCAINE HYDROCHLORIDE (International Medication Systems), LIDOCAINE HYDROCHLORIDE (International Medication Systems), LIDOCAINE HYDROCHLORIDE (Lannett Company), LIDOCAINE HYDROCHLORIDE (Lifestar Pharma LLC), LIDOCAINE HYDROCHLORIDE (Lifestar Pharma LLC), LIDOCAINE HYDROCHLORIDE (Marlex Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (McKesson Corporation dba SKY Packaging), LIDOCAINE HYDROCHLORIDE (McKesson Corporation dba SKY Packaging), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (Medical Purchasing Solutions), LIDOCAINE HYDROCHLORIDE (NorthStar RxLLC), LIDOCAINE HYDROCHLORIDE (NorthStar RxLLC), LIDOCAINE HYDROCHLORIDE (Novagenix Labs LLC), LIDOCAINE HYDROCHLORIDE (Novitium Pharma LLC), LIDOCAINE HYDROCHLORIDE (NuCare Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (NuCare Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (NuCare Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (PAI Holdings), LIDOCAINE HYDROCHLORIDE (PAI Holdings), LIDOCAINE HYDROCHLORIDE (Precision Dose), LIDOCAINE HYDROCHLORIDE (Preferred Pharmaceuticals), LIDOCAINE HYDROCHLORIDE (REMEDYREPACK), LIDOCAINE HYDROCHLORIDE (REMEDYREPACK), LIDOCAINE HYDROCHLORIDE (Spectra Medical Devices), LIDOCAINE HYDROCHLORIDE (The J. Molner Company LLC), LIDOCAINE HYDROCHLORIDE (Wockhardt USA LLC.), LIDOCAINE VISCOUS (A-S Medication Solutions), LIDOCAINE VISCOUS (Cardinal Health 107), LIDOCAINE VISCOUS (Hikma Pharmaceuticals USA), LIDOCAINE VISCOUS (Pharmaceutical Associates), NA (SINTETICA SA), NA (Sintetica US LLC) |
|---|
| 組成式 |
C14H22N2O. HCl
|
|---|
| 質量 |
270.1499
|
|---|
| 分子量 |
270.80
|
|---|
| 構造式 |
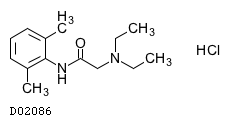
|
|---|
| Simcomp |
|
|---|
| クラス |
|
|---|
| コード |
| 商品 (DG00196): | D00358<JP/US> D02086<JP/US> D08127<JP/US> |
| 商品 (mixture): | D04052<JP/US> D04813<JP/US> |
|
|---|
| 効能 |
局所麻酔薬, 抗不整脈薬, ナトリウムチャネル遮断薬 |
|---|
| コメント |
アニリド系麻酔薬
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 代謝 |
Enzyme: CYP1A2 [HSA: 1544], CYP3A4 [HSA: 1576]
|
|---|
| 相互作用 |
|
|---|
| 構造マップ |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
1 神経系及び感覚器官用医薬品
12 末梢神経系用薬
121 局所麻酔剤
1214 キシリジン系製剤
D02086 リドカイン塩酸塩 (JAN); 塩酸リドカイン
13 感覚器官用薬
131 眼科用剤
1313 眼科用局所麻酔剤
D02086 リドカイン塩酸塩 (JAN); 塩酸リドカイン
2 個々の器官系用医薬品
21 循環器官用薬
212 不整脈用剤
2129 その他の不整脈用剤
D02086 リドカイン塩酸塩 (JAN); 塩酸リドカイン
医療用医薬品のATC分類 [BR:jp08303]
C 循環器系
C01 心疾患治療
C01B 抗不整脈薬、クラスIとIII
C01BB 抗不整脈薬、クラスIb
C01BB01 リドカイン
D02086 リドカイン塩酸塩 (JAN) <JP/US>
C05 血管保護薬
C05A 痔核と裂肛の局所用治療薬
C05AD 局所麻酔薬
C05AD01 リドカイン
D02086 リドカイン塩酸塩 (JAN) <JP/US>
D 皮膚科用薬
D04 鎮痒薬、抗ヒスタミン薬・麻酔薬等を含む
D04A 鎮痒薬、抗ヒスタミン薬・麻酔薬等を含む
D04AB 局所用麻酔薬
D04AB01 リドカイン
D02086 リドカイン塩酸塩 (JAN) <JP/US>
N 神経系
N01 麻酔薬
N01B 麻酔薬、局所
N01BB アミド
N01BB02 リドカイン
D02086 リドカイン塩酸塩 (JAN) <JP/US>
R 呼吸器系
R02 咽頭用製剤
R02A 咽頭用製剤
R02AD 麻酔薬、局所
R02AD02 リドカイン
D02086 リドカイン塩酸塩 (JAN) <JP/US>
S 感覚器
S01 眼科用薬
S01H 局所麻酔薬
S01HA 局所麻酔薬
S01HA07 リドカイン
D02086 リドカイン塩酸塩 (JAN) <JP/US>
S02 耳科用薬
S02D その他の耳科用薬
S02DA 鎮痛薬と麻酔薬
S02DA01 リドカイン
D02086 リドカイン塩酸塩 (JAN) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
麻酔薬
局所麻酔薬
リドカイン
D02086 リドカイン塩酸塩 (JAN)
一般用医薬品の分類 [BR:jp08313]
泌尿生殖器官及び肛門用薬
30 外用痔疾用薬
D02086 リドカイン塩酸塩 (JAN)
外皮用薬
57 鎮痛・鎮痒・収れん・消炎薬(パップ剤を含む)
D02086 リドカイン塩酸塩 (JAN)
一般用医薬品のリスク区分 [BR:jp08312]
第二類医薬品
無機薬品及び有機薬品
リドカイン
D02086 リドカイン塩酸塩 (JAN)
医薬品グループ [BR:jp08330]
心血管系用薬
DG01653 抗不整脈薬
DG01652 クラスI抗不整脈薬
DG01651 クラスIb抗不整脈薬
DG00196 リドカイン
D02086 リドカイン塩酸塩
精神神経系用薬
DG02030 麻酔薬
DG01675 局所麻酔薬
DG01673 アミド型局所麻酔薬
DG00196 リドカイン
D02086 リドカイン塩酸塩
代謝酵素基質薬
DG01892 CYP1A2基質薬
DG00196 リドカイン
D02086 リドカイン塩酸塩
DG01633 CYP3A/CYP3A4基質薬
DG02913 CYP3A4基質薬
DG00196 リドカイン
D02086 リドカイン塩酸塩
医薬品クラス [BR:jp08332]
心血管系用薬
DG01653 抗不整脈薬 [商品一覧]
D02086 リドカイン塩酸塩
ターゲットに基づく医薬品分類 [BR:jp08310]
イオンチャネル
電位依存性イオンチャネル
ナトリウムチャネル
SCN1A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN2A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN3A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN4A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN5A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN8A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN9A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN10A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
SCN11A
D02086 リドカイン塩酸塩 (JAN) <JP/US>
薬物代謝酵素とトランスポーター [jp08309.html]
薬物代謝酵素
D02086
医薬品グループ [BR:jp08330]
心血管系用薬
DG01653 抗不整脈薬
DG01652 クラスI抗不整脈薬
DG01651 クラスIb抗不整脈薬
DG00196 リドカイン
精神神経系用薬
DG02030 麻酔薬
DG01675 局所麻酔薬
DG01673 アミド型局所麻酔薬
DG00196 リドカイン
代謝酵素基質薬
DG01892 CYP1A2基質薬
DG00196 リドカイン
DG01633 CYP3A/CYP3A4基質薬
DG02913 CYP3A4基質薬
DG00196 リドカイン
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|