 | | DRUG: タダラフィル | |
| エントリ |
|
|---|
| 一般名 |
タダラフィル (JAN);
Tadalafil (JAN/USP/INN) |
|---|
| 商品名 |
アドシルカ (日本新薬), ザルティア (日本新薬), シアリス (日本新薬), タダラフィル (キョーリンリメディオ), タダラフィル (シオノケミカル), タダラフィル (リョートーファイン), タダラフィル (大興製薬), タダラフィル (富士化学工業), タダラフィル (東和薬品), タダラフィル (江州製薬), タダラフィル (沢井製薬), タダラフィル (辰巳化学), タダラフィル (辰巳化学) |
|---|
| 後発品 |
タダラフィル (あすか製薬), タダラフィル (キョーリンリメディオ), タダラフィル (キョーリンリメディオ), タダラフィル (キョーリンリメディオ), タダラフィル (キョーリンリメディオ), タダラフィル (サンド), タダラフィル (シオエ製薬), タダラフィル (シオノケミカル), タダラフィル (トーアエイヨー), タダラフィル (ニプロ), タダラフィル (日医工), タダラフィル (日本ジェネリック), タダラフィル (日本ジェネリック), タダラフィル (東和薬品), タダラフィル (東和薬品), タダラフィル (東和薬品), タダラフィル (沢井製薬), タダラフィル (沢井製薬) |
|---|
| 米国の商品 |
|
|---|
| 後発品 |
ALYQ (Teva Pharmaceuticals), N/A (AustarPharma LLC), TADALAFIL (A-S Medication Solutions), TADALAFIL (A-S Medication Solutions), TADALAFIL (A-S Medication Solutions), TADALAFIL (A-S Medication Solutions), TADALAFIL (A-S Medication Solutions), TADALAFIL (A-S Medication Solutions), TADALAFIL (A-S Medication Solutions), TADALAFIL (A-S Medication Solutions), TADALAFIL (ANI Pharmaceuticals), TADALAFIL (Accord Healthcare), TADALAFIL (Advanced Rx of Tennessee), TADALAFIL (Ajanta Pharma USA), TADALAFIL (Ajanta Pharma USA), TADALAFIL (Alembic Pharmaceuticals Limited), TADALAFIL (Alembic Pharmaceuticals), TADALAFIL (Amneal Pharmaceuticals NY LLC), TADALAFIL (Amneal Pharmaceuticals NY LLC), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Aphena Pharma Solutions - Tennessee), TADALAFIL (Asclemed USA), TADALAFIL (Asclemed USA), TADALAFIL (Asclemed USA), TADALAFIL (Asclemed USA), TADALAFIL (Asclemed USA), TADALAFIL (Asclemed USA), TADALAFIL (Aurobindo Pharma Limited), TADALAFIL (Aurobindo Pharma Limited), TADALAFIL (AvKARE), TADALAFIL (AvKARE), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Bryant Ranch Prepack), TADALAFIL (Burel Pharmaceuticals), TADALAFIL (Camber Pharmaceuticals), TADALAFIL (Camber Pharmaceuticals), TADALAFIL (Cipla USA), TADALAFIL (Coupler LLC), TADALAFIL (Dr.Reddys Laboratories), TADALAFIL (Dr.Reddys Laboratories), TADALAFIL (FOURRTS (INDIA) LABORATORIES PRIVATE LIMITED), TADALAFIL (Lupin Pharmaceuticals), TADALAFIL (Macleods Pharmaceuticals Limited), TADALAFIL (Macleods Pharmaceuticals Limited), TADALAFIL (Medsource Pharmaceuticals), TADALAFIL (NIVAGEN PHARMACEUTICALS), TADALAFIL (NorthStar RxLLC), TADALAFIL (Northwind Pharmaceuticals), TADALAFIL (Northwind Pharmaceuticals), TADALAFIL (Northwind Pharmaceuticals), TADALAFIL (Northwind Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (NuCare Pharmaceuticals), TADALAFIL (PD-RX Pharmaceuticals), TADALAFIL (PD-Rx Pharmaceuticals), TADALAFIL (PD-Rx Pharmaceuticals), TADALAFIL (PD-Rx Pharmaceuticals), TADALAFIL (PD-Rx Pharmaceuticals), TADALAFIL (PD-Rx Pharmaceuticals), TADALAFIL (PD-Rx Pharmaceuticals), TADALAFIL (Preferred Pharmaceuticals), TADALAFIL (Preferred Pharmaceuticals), TADALAFIL (Preferred Pharmaceuticals), TADALAFIL (Preferred Pharmaceuticals), TADALAFIL (Preferred Pharmaceuticals), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Proficient Rx LP), TADALAFIL (Qilu Pharmaceutical), TADALAFIL (Quallent Pharmaceuticals Health LLC), TADALAFIL (Quallent Pharmaceuticals Health LLC), TADALAFIL (REMEDYREPACK), TADALAFIL (REMEDYREPACK), TADALAFIL (REMEDYREPACK), TADALAFIL (REMEDYREPACK), TADALAFIL (REMEDYREPACK), TADALAFIL (Solco Healthcare US), TADALAFIL (Solco Healthcare US), TADALAFIL (Solco Healthcare US), TADALAFIL (Sun Pharmaceutical Industries), TADALAFIL (Teva Pharmaceuticals), TADALAFIL (Torrent Pharmaceuticals Limited), TADALAFIL (Torrent Pharmaceuticals Limited), TADALAFIL (Unichem Pharmaceuticals (USA)), TADALAFIL (Upsher-Smith Laboratories), TADALAFIL (VKT Pharma Private Limited), TADALAFIL (XLCare Pharmaceuticals), TADALAFIL (Zydus Lifesciences Limited), TADALAFIL (Zydus Pharmaceuticals USA), TADALAFIL (direct rx) |
|---|
| 組成式 |
C22H19N3O4
|
|---|
| 質量 |
389.1376
|
|---|
| 分子量 |
389.40
|
|---|
| 構造式 |
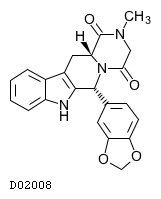
|
|---|
| クラス |
|
|---|
| コード |
| 商品 (mixture): | D12963<JP/US> |
|
|---|
| 効能 |
血圧降下薬, 勃起不全治療薬, ホスホジエステラーゼV阻害薬 |
|---|
| 疾患 |
|
|---|
| ターゲット |
|
|---|
| パスウェイ |
|
|---|
| 代謝 |
Enzyme: CYP3A4 [HSA: 1576]
|
|---|
| 相互作用 |
|
|---|
| 階層分類 |
医療用医薬品の薬効分類 [BR:jp08301]
2 個々の器官系用医薬品
21 循環器官用薬
219 その他の循環器官用薬
2190 その他の循環器官用薬
D02008 タダラフィル (JAN)
25 泌尿生殖器官及び肛門用薬
259 その他の泌尿生殖器官及び肛門用薬
2590 その他の泌尿生殖器官及び肛門用薬
D02008 タダラフィル (JAN)
医療用医薬品のATC分類 [BR:jp08303]
G 泌尿生殖器系と性ホルモン
G04 泌尿器科用薬
G04B 泌尿器科用薬
G04BE 勃起不全用薬
G04BE08 タダラフィル
D02008 タダラフィル (JAN) <JP/US>
医療用医薬品のUSP分類 [BR:jp08302]
泌尿生殖器薬
前立腺肥大症治療薬
タダラフィル
D02008 タダラフィル (JAN)
呼吸器薬
肺血圧降下薬
ホスホジエステラーゼ-5 (PDE5)阻害薬
タダラフィル
D02008 タダラフィル (JAN)
性的障害治療薬
性的障害治療薬(男性)
タダラフィル
D02008 タダラフィル (JAN)
医薬品グループ [BR:jp08330]
心血管系用薬
DG01509 ホスホジエステラーゼV阻害薬
D02008 タダラフィル
代謝酵素基質薬
DG01633 CYP3A/CYP3A4基質薬
DG02913 CYP3A4基質薬
D02008 タダラフィル
ターゲットに基づく医薬品分類 [BR:jp08310]
酵素
ヒドロラーゼ (EC3)
ホスホジエステラーゼ
PDE5
D02008 タダラフィル (JAN) <JP/US>
日本の新薬 [jp08318.html]
新有効成分含有医薬品
D02008
日本・米国・欧州の新薬 [jp08328.html]
日米欧での承認状況
D02008
薬物代謝酵素とトランスポーター [jp08309.html]
薬物代謝酵素
D02008
|
|---|
| リンク |
|
|---|
| LinkDB |
|
|---|
|
» English version » Back
|