 | | PATHWAY: ko03230 | |
| Entry |
|
|---|
| Name |
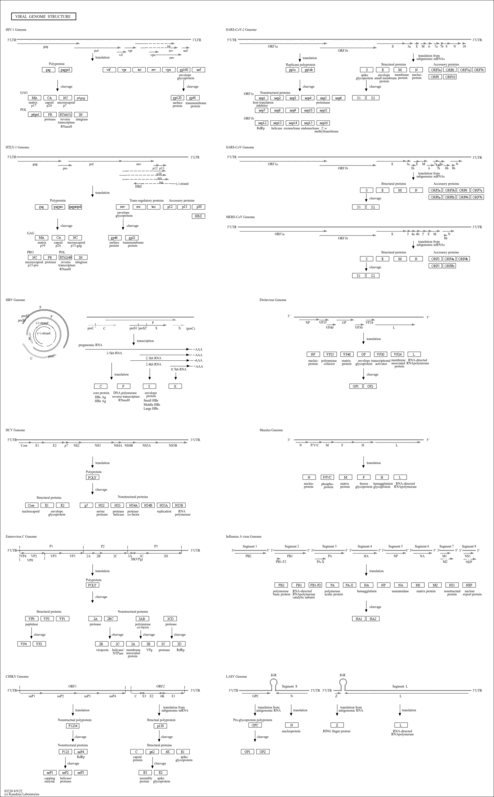
Viral genome structure |
|---|
| Class |
Genetic Information Processing; Information processing in viruses
|
|---|
| Pathway map |
|
|---|
| Drug |
| D00296 | Didanosine (JAN/USP/INN) |
| D00353 | Lamivudine (JAN/USP/INN) |
| D00412 | Zalcitabine (JAN/USP/INN) |
| D00413 | Zidovudine (JP18/USP/INN) |
| D00423 | Ribavirin (JP18/USP/INN) |
| D00427 | Ritonavir (JAN/USP/INN) |
| D00429 | Saquinavir (JAN/USP/INN) |
| D00435 | Nevirapine (JAN/USP/INN) |
| D00777 | Amantadine hydrochloride (JP18/USP) |
| D00891 | Abacavir sulfate (JAN/USP) |
| D00894 | Amprenavir (JAN/USAN/INN) |
| D00895 | Delavirdine mesylate (USAN) |
| D00896 | Efavirenz (JAN/USP/INN) |
| D00897 | Indinavir sulfate (USP) |
| D00899 | Nelfinavir mesylate (USAN/INN) |
| D00900 | Oseltamivir phosphate (JAN/USP) |
| D00901 | Rimantadine hydrochloride (USP) |
| D01160 | Saquinavir mesylate (USP) |
| D01199 | Emtricitabine (JAN/USAN/INN) |
| D01276 | Atazanavir sulfate (JAN/USAN) |
| D01425 | Lopinavir (JAN/USP/INN) |
| D01655 | Adefovir dipivoxil (USAN) |
| D01937 | Zanamivir hydrate (JAN) |
| D01982 | Tenofovir disoproxil fumarate (JAN/USAN) |
| D02297 | Emtricitabine and tenofovir disoproxil |
| D02498 | Lopinavir and ritonavir |
| D02734 | Abacavir succinate (USAN) |
| D02861 | Indinavir sulfate ethanolate (JAN) |
| D02867 | Fosamprenavir calcium hydrate (JAN) |
| D03001 | Atevirdine mesylate (USAN) |
| D03433 | Celgosivir hydrochloride (USAN) |
| D03506 | Cinanserin hydrochloride (USAN) |
| D03835 | Fosamprenavir calcium (USAN) |
| D03837 | Fosamprenavir sodium (USAN) |
| D03839 | Droxinavir hydrochloride (USAN) |
| D03843 | Tipranavir disodium (USAN) |
| D03981 | Elvucitabine (USAN/INN) |
| D04112 | Etravirine (JAN/USAN/INN) |
| D05588 | Pradefovir mesylate (USAN) |
| D06207 | Trecovirsen sodium (USAN) |
| D06278 | Valtorcitabine dihydrochloride (USAN) |
| D06478 | Darunavir ethanolate (JAN) |
| D06564 | Bevirimat dimeglumine (USAN) |
| D06580 | Dexelvucitabine (USAN/INN) |
| D06651 | Taribavirin hydrochloride (USAN) |
| D06677 | Elvitegravir (JAN/USAN) |
| D07133 | Raltegravir potassium (JAN/USAN) |
| D07507 | Zidovudine and lamivudine |
| D08775 | Abacavir sulfate and lamivudine |
| D09012 | Telaprevir (JAN/USAN/INN) |
| D09028 | Valopicitabine dihydrochloride (USAN) |
| D09537 | Favipiravir (JAN/USAN/INN) |
| D09547 | Laninamivir octanoate hydrate (JAN) |
| D09583 | Balapiravir hydrochloride (USAN) |
| D09720 | Rilpivirine (JAN/USAN/INN) |
| D09885 | Danoprevir sodium (USAN) |
| D09958 | Rilpivirine hydrochloride (JAN/USAN) |
| D09987 | Vaniprevir (JAN/USAN/INN) |
| D10105 | Daclatasvir dihydrochloride (USAN) |
| D10113 | Dolutegravir sodium (JAN/USAN) |
| D10366 | Sofosbuvir (JAN/USAN/INN) |
| D10394 | Deldeprevir sodium (USAN) |
| D10428 | Tenofovir alafenamide (USAN/INN) |
| D10462 | Faldaprevir sodium (JAN/USAN) |
| D10469 | Simeprevir sodium (JAN) |
| D10477 | Mericitabine (USAN/INN) |
| D10548 | Cabotegravir (JAN/USAN/INN) |
| D10549 | Cabotegravir sodium (JAN/USAN) |
| D10571 | Rilpivirine hydrochloride, tenofovir disoproxil fumarate and emtricitabine |
| D10578 | Ledipasvir and sofosbuvir |
| D10580 | Paritaprevir (USAN/INN) |
| D10581 | Dasabuvir sodium hydrate |
| D10582 | Dasabuvir, ombitasvir, paritaprevir and ritonavir |
| D10597 | Paritaprevir hydrate (JAN) |
| D10598 | Ombitasvir hydrate (JAN) |
| D10599 | Ledipasvir acetonate (JAN) |
| D10600 | Dolutegravir, abacavir and lamivudine |
| D10605 | Tenofovir alafenamide fumarate (JAN/USAN) |
| D10611 | Beclabuvir hydrochloride (JAN/USAN) |
| D10622 | Deleobuvir sodium (JAN/USAN) |
| D10624 | Doravirine (JAN/USAN/INN) |
| D10625 | Elbasvir (JAN/USAN/INN) |
| D10708 | Fostemsavir tromethamine (USAN) |
| D10745 | Ombitasvir, paritaprevir and ritonavir |
| D10753 | Atazanavir and cobicistat |
| D10754 | Lamivudine and raltegravir |
| D10755 | Elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide |
| D10756 | Elvitegravir, cobicistat, emtricitabine and tenofovir disoproxil |
| D10778 | Elbasvir and grazoprevir |
| D10806 | Velpatasvir (JAN/USAN/INN) |
| D10815 | Glecaprevir hydrate (JAN) |
| D10816 | Pibrentasvir (JAN/USAN/INN) |
| D10832 | Darunavir and cobicistat |
| D10834 | Tenofovir disoproxil phosphate |
| D10835 | Emtricitabine and tenofovir alafenamide |
| D10838 | Abacavir, lamivudine and zidovudine |
| D10851 | Efavirenz, emtricitabine and tenofovir disoproxil |
| D10882 | Daclatasvir, asunaprevir and beclabuvir |
| D10899 | Voxilaprevir (USAN/INN) |
| D10900 | Sofosbuvir, velpatasvir and voxilaprevir |
| D10910 | Bictegravir sodium (JAN/USAN) |
| D10919 | Cosfroviximab (USAN/INN) |
| D10948 | Larcaviximab (USAN/INN) |
| D10974 | Porgaviximab (USAN/INN) |
| D10996 | Uprifosbuvir (USAN/INN) |
| D11021 | Baloxavir marboxil (JAN/USAN/INN) |
| D11039 | Bictegravir, emtricitabine and tenofovir alafenamide |
| D11282 | Dolutegravir sodium and rilpivirine hydrochloride |
| D11367 | Pimodivir hydrochloride (USAN) |
| D11382 | Darunavir, cobicistat, emtricitabine and tenofovir alafenamide |
| D11391 | Tenofovir disoproxil maleate |
| D11392 | Efavirenz, lamivudine and tenofovir disoproxil fumarate |
| D11395 | Lamivudine and tenofovir disoproxil |
| D11396 | Doravirine, lamivudine and tenofovir disoproxil |
| D11521 | Lamivudine, nevirapine and zidovudine |
| D11522 | Dolutegravir and lamivudine |
| D11721 | Rovafovir etalafenamide (USAN) |
| D11722 | Rovafovir etalafenamide citrate (USAN) |
| D11914 | Islatravir hydrate (JAN/USAN) |
| D11922 | Atoltivimab, maftivimab and odesivimab |
| D11943 | Molnupiravir (JAN/USAN) |
| D11966 | Cabotegravir and rilpivirine |
| D12115 | COVID-19 vaccine (ChAdOx1-S [recombinant]) |
| D12126 | Bersacapavir (USAN/INN) |
| D12129 | COVID-19 vaccine (Ad26.COV2-S [recombinant]) |
| D12142 | Casirivimab and imdevimab |
| D12162 | Lenacapavir sodium (JAN/USAN) |
| D12244 | Nirmatrelvir (JAN/USAN) |
| D12260 | Bemnifosbuvir sulfate (USAN) |
| D12262 | Tixagevimab and cilgavimab |
| D12336 | SARS-CoV-2 Spike glycoprotein vaccine antigen nvx-cov2373 |
| D12353 | Ensitrelvir fumarate (USAN) |
| D12389 | Adintrevimab (USAN/INN) |
| D12503 | Linvencorvir (USAN/INN) |
| D12523 | Fipravirimat (USAN/INN) |
| D12535 | Fipravirimat mesylate (USAN) |
| D12745 | Ravidasvir hydrochloride (USAN) |
| D12752 | Darunavir propylene glycolate |
| D12776 | Obeldesivir (JAN/USAN/INN) |
| D12778 | Olgotrelvir sodium (USAN) |
| D12944 | Zegruvirimat (USAN/INN) |
| D12945 | Zegruvirimat tosylate (USAN) |
|
|---|
| Orthology |
| K00985 | 3D; Enterovirus RNA-directed RNA polymerase |
| K01516 | 2C; Enterovirus protein 2C |
| K19251 | HA; Influenza A virus hemagglutinin |
| K19259 | env; Deltaretrovirus envelope glycoprotein gp62 |
| K19287 | env, gp160; HIV envelope glycoprotein gp160 |
| K19288 | p130; Alphavirus structral polyprotein |
| K19358 | HCVgp1; Hepatitis C virus polyprotein |
| K19377 | P, P/V/C; Morbillivirus phosphoprotein |
| K19378 | H; Morbillivirus hemagglutinin glycoprotein |
| K19379 | F; Morbillivirus fusion glycoprotein F0 |
| K19380 | N; Morbillivirus nucleoprotein |
| K19381 | M; Morbillivirus matrix protein |
| K19388 | PB2; Influenza virus polymerase basic protein 2 |
| K19389 | PB1; Influenza virus RNA-directed RNA polymerase catalytic subunit [EC:2.7.7.48] |
| K19390 | PA; Influenza virus polymerase acidic protein |
| K19391 | NP; Influenza virus nucleoprotein |
| K19393 | M1; Influenza A/B virus matrix protein 1 |
| K19394 | M2; Influenza A virus matrix protein 2 |
| K19395 | NEP, NS2; Influenza A virus nuclear export protein |
| K19396 | NS1; Influenza A virus non-structural protein 1 |
| K19397 | PB1F2; Influenza A virus protein PB1-F2 |
| K21854 | X; Orthohepadnavirus protein X |
| K21857 | tax; Deltaretrovirus protein Tax-1 |
| K22202 | vpr; HIV virus protein R |
| K22271 | Core; Hepatitis C virus core protein |
| K22272 | E2; Hepatitis C virus envelope glycoprotein E2 |
| K22274 | NS4A; Hepatitis C virus non-structural protein 4A |
| K22275 | NS5A; Hepatitis C virus non-structural protein 5A |
| K22472 | NS5B; Hepatitis C virus RNA-dependent RNA polymerase [EC:2.7.7.48] |
| K22497 | NS2; Hepatitis C virus non-structural protein 2 [EC:3.4.22.-] |
| K22498 | NS4B; Hepatitis C virus non-structural protein 4B |
| K22892 | vif; HIV virion infectivity factor |
| K22951 | gag, gag-pol; HIV Gag or Gag-Pol polyprotein |
| K23017 | gag; Deltaretrovirus gag polyprotein |
| K23018 | rex; HTLV-1 rex polyprotein |
| K23068 | p30II; HTLV-1 accessory protein p30II |
| K23069 | p12I; HTLV-1 accessory protein p12I |
| K23070 | p13; HTLV-1 accessory protein p13 |
| K23071 | HBZ; HTLV-1 basic zipper factor |
| K23128 | S; Orthohepadnavirus large envelope protein |
| K23129 | C; Orthohepadnavirus capsid protein |
| K23454 | gag-pro-pol; Deltaretrovirus gag-pro-pol polyprotein |
| K24106 | N; SARS coronavirus nucleocapsid protein |
| K24149 | pp1ab; Coronaviridae replicase polyprotein 1ab |
| K24150 | M; SARS coronavirus membrane protein |
| K24151 | E; SARS coronavirus envelope small membrane protein |
| K24152 | S; SARS coronavirus spike glycoprotein |
| K24266 | gp120; HIV envelope glycoprotein gp120 |
| K24320 | GPC; Old World arenavirus glycoprotein |
| K24324 | S; MERS coronavirus spike glycoprotein |
| K24534 | GP; Ebolavirus envelope glycoprotein |
| K24579 | 3a; SARS coronavirus accessory protein 3a |
| K24580 | 6; SARS coronavirus accessory protein 6 |
| K24581 | 7a; SARS coronavirus accessory protein 7a |
| K24582 | 7b; SARS coronavirus accessory protein 7b |
| K24583 | 8; SARS coronavirus accessory protein 8 |
| K24584 | 10; SARS coronavirus accessory protein 10 |
| K24801 | gp41; HIV transmembrane fusion protein gp41 |
| K24802 | RT; HIV-1 reverse transcriptase / ribonuclease H |
| K24861 | E1; Hepatitis C virus envelope glycoprotein E1 |
| K24862 | gp46; HTLV envelope surface glycoprotein gp46 |
| K24863 | gp21; HTLV envelope transmembrane protein gp21 |
| K25001 | S1; SARS coronavirus spike protein S1 |
| K25002 | S2; SARS coronavirus spike protein S2 |
| K25011 | nsp1; SARS coronavirus host translation inhibitor nsp1 |
| K25012 | nsp2; SARS coronavirus non-structural protein 2 |
| K25013 | nsp3; SARS coronavirus non-structural protein 3 |
| K25014 | nsp4; SARS coronavirus non-structural protein 4 |
| K25015 | nsp5; SARS coronavirus 3C-like proteinase |
| K25016 | nsp6; SARS coronavirus non-structural protein 6 |
| K25017 | nsp7; SARS coronavirus non-structural protein 7 |
| K25018 | nsp8; SARS coronavirus non-structural protein 8 |
| K25019 | nsp9; SARS coronavirus non-structural protein 9 |
| K25020 | nsp10; SARS coronavirus non-structural protein 10 |
| K25021 | nsp12; Coronaviridae RNA-directed RNA polymerase |
| K25022 | nsp13; SARS coronavirus helicase |
| K25023 | nsp14; SARS coronavirus proofreading exoribonuclease |
| K25024 | nsp15; SARS coronavirus uridylate-specific endoribonuclease |
| K25025 | nsp16; SARS coronavirus 2'-O-methyltransferase |
| K25051 | MA; HTLV-1 matrix protein |
| K25052 | CA; HTLV-1 capsid protein |
| K25053 | NC; HTLV-1 nucleocapsid protein |
| K25055 | RT; HTLV-1 reverse transcriptase / ribonuclease H |
| K25125 | GP1; Old World arenavirus glycoprotein GP1 |
| K25126 | E2; Alphavirus spike glycoprotein E2 |
| K25127 | VP1; Enterovirus C capsid protein VP1 |
| K25146 | PA-X; Influenza A virus protein PA-X |
| K25160 | HA1; Influenza A virus hemagglutinin HA1 chain |
| K25161 | HA2; Influenza A virus hemagglutinin HA2 chain |
| K25281 | gp1; Enterovirus genome polyprotein |
| K25293 | 2A; Enterovirus protease 2A |
| K25294 | 2B; Enterovirus protein 2B |
| K25295 | 3AB; Enterovirus protein 3AB |
| K25296 | 3A; Enterovirus protein 3A |
| K25297 | 3B; Enterovirus viral protein genome-linked |
| K25298 | 3CD; Enterovirus protein 3CD |
| K25299 | 3C; Enterovirus protease 3C |
| K25300 | gp1; Alphavirus polyprotein P1234 |
| K25301 | N; Mammarenavirus nucleoprotein |
| K25302 | Z; Mammarenavirus RING finger protein Z |
| K25303 | L; Mammarenavirus RNA-directed RNA polymerase L |
| K25319 | GP2; Mammarenavirus glycoprotein GP2 |
| K25333 | L; Ebolavirus RNA-directed RNA polymerase L |
| K25334 | VP35; Ebolavirus polymerase cofactor VP35 |
| K25335 | NP; Ebolavirus nucleoprotein |
| K25804 | E1; Alphavirus spike glycoprotein E1 |
| K25928 | VP0; Enterovirus capsid protein VP0 |
|
|---|
| Reference |
|
|---|
| Authors |
de Oliveira T, Engelbrecht S, Janse van Rensburg E, Gordon M, Bishop K, zur Megede J, Barnett SW, Cassol S |
|---|
| Title |
Variability at human immunodeficiency virus type 1 subtype C protease cleavage sites: an indication of viral fitness? |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Hayes AM, Qian S, Yu L, Boris-Lawrie K |
|---|
| Title |
Tat RNA silencing suppressor activity contributes to perturbation of lymphocyte miRNA by HIV-1. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Zhang LL, Wei JY, Wang L, Huang SL, Chen JL |
|---|
| Title |
Human T-cell lymphotropic virus type 1 and its oncogenesis. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Al-Sadeq DW, Taleb SA, Zaied RE, Fahad SM, Smatti MK, Rizeq BR, Al Thani AA, Yassine HM, Nasrallah GK |
|---|
| Title |
Hepatitis B Virus Molecular Epidemiology, Host-Virus Interaction, Coinfection, and Laboratory Diagnosis in the MENA Region: An Update. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Tellinghuisen TL, Evans MJ, von Hahn T, You S, Rice CM |
|---|
| Title |
Studying hepatitis C virus: making the best of a bad virus. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Alanagreh L, Alzoughool F, Atoum M |
|---|
| Title |
The Human Coronavirus Disease COVID-19: Its Origin, Characteristics, and Insights into Potential Drugs and Its Mechanisms. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Polycarpou A, Howard M, Farrar CA, Greenlaw R, Fanelli G, Wallis R, Klavinskis LS, Sacks S |
|---|
| Title |
Rationale for targeting complement in COVID-19. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Krishnamoorthy S, Swain B, Verma RS, Gunthe SS |
|---|
| Title |
SARS-CoV, MERS-CoV, and 2019-nCoV viruses: an overview of origin, evolution, and genetic variations. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Singh RK, Dhama K, Malik YS, Ramakrishnan MA, Karthik K, Khandia R, Tiwari R, Munjal A, Saminathan M, Sachan S, Desingu PA, Kattoor JJ, Iqbal HM, Joshi SK |
|---|
| Title |
Ebola virus - epidemiology, diagnosis, and control: threat to humans, lessons learnt, and preparedness plans - an update on its 40 year's journey. |
|---|
| Journal |
|
|---|
| Reference |
|
|---|
| Authors |
Bankamp B, Takeda M, Zhang Y, Xu W, Rota PA |
|---|
| Title |
Genetic characterization of measles vaccine strains. |
|---|
| Journal |
|
|---|
|
|